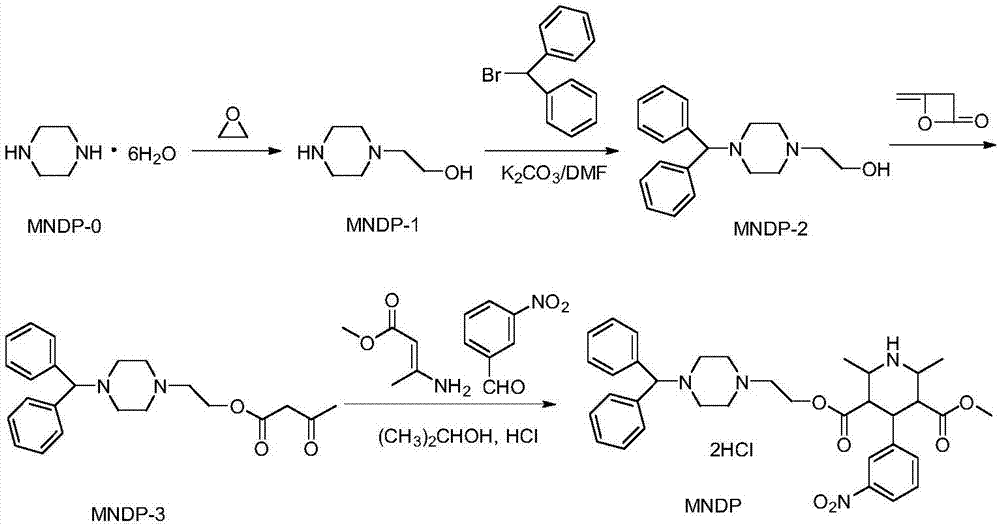
Preparation method of manidipine hydrochloride
A technology of manidipine hydrochloride and ethyl acetoacetate, which is applied in the field of preparation of manidipine hydrochloride, can solve the problems of low recovery rate, complex preparation method of manidipine hydrochloride, low product purity, etc., and achieve low cost, The effect of mild reaction conditions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
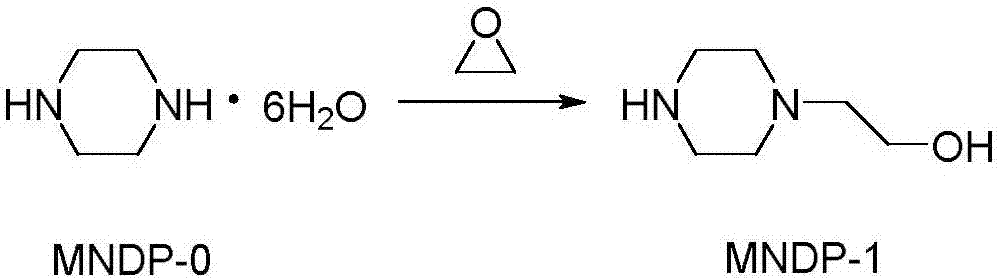
[0035] 1) Synthesis of N-(2-hydroxyethyl)piperazine
[0036]Add 194g of piperazine (MNDP-0) and 400ml of water into the reaction flask, cool in an ice bath to 15-20°C, add 26.5g of ethylene oxide, stir and react at 30-35°C for 2 hours, then slowly heat to Temperature 108-110°C, stop the reaction when 360ml of water is distilled, cool to 10°C, filter to remove unreacted piperazine after standing still, distill the filtrate under reduced pressure, collect fractions at 125-127°C / 1.6kPa to obtain off-white viscous oil 1-Benzhydryl-4-(2-hydroxyethyl)piperazine 48.4g (namely MNDP-1), yield 81.7%, such as figure 2 shown.
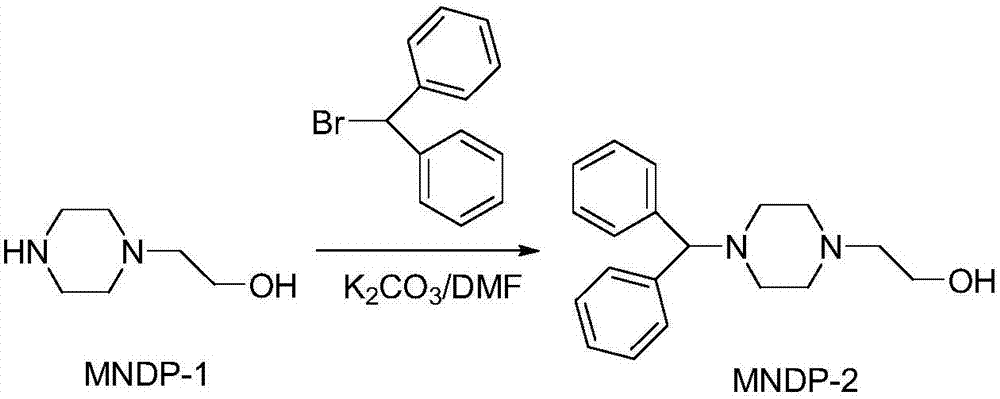
[0037] 2) Synthesis of 1-benzhydryl-4-(2-hydroxyethyl)piperazine
[0038] Add 48.4g of MNDP-1, 51.5g of anhydrous potassium carbonate powder and 500ml of DMF into the reaction flask, add 91.4g of benzhydryl bromide in 3 batches under stirring, stir at room temperature for 3.5 hours, transfer to 1000ml of water, and extract with ether. The ether phase was washed...
Embodiment 2
[0044] A preparation method of manidipine hydrochloride, comprising the following steps:
[0045] (1) Synthesis of N-(2-hydroxyethyl)piperazine, piperazine and water were added to the reaction flask, cooled to 18°C in an ice bath, added ethylene oxide, stirred and reacted at 32°C for 2 hours, then slowly Heat to a temperature of 109°C, stop the reaction when 90% of water is distilled, cool to 10°C, filter to remove unreacted piperazine after standing still, distill the filtrate under reduced pressure, collect when the temperature is 126°C and the pressure is 1.6kPa The fraction of N-(2-hydroxyethyl)piperazine is obtained as gray-white viscous oil, such as figure 2 shown;
[0046] (2) Synthesis of 1-benzhydryl-4-(2-hydroxyethyl) piperazine, N-(2-hydroxyethyl) piperazine, anhydrous potassium carbonate powder and DMF are added in the reaction flask, stirred Add benzhydryl bromide in 3 batches, stir at room temperature for 3.5 hours, add water, extract with ether, wash the et...
Embodiment 3
[0054] A preparation method of manidipine hydrochloride, comprising the following steps:
[0055] (1) Synthesis of N-(2-hydroxyethyl)piperazine, piperazine and water were added to the reaction flask, cooled to 18°C in an ice bath, added ethylene oxide, stirred and reacted at 32°C for 2 hours, then slowly Heat to a temperature of 108°C, stop the reaction when 90% of water is distilled, cool to 10°C, filter to remove unreacted piperazine after standing still, distill the filtrate under reduced pressure, collect when the temperature is 125°C and the pressure is 1.6kPa The fraction of N-(2-hydroxyethyl)piperazine is obtained as gray-white viscous oil, such as figure 2 shown;
[0056] (2) Synthesis of 1-benzhydryl-4-(2-hydroxyethyl) piperazine, N-(2-hydroxyethyl) piperazine, anhydrous potassium carbonate powder and DMF are added in the reaction flask, stirred Add benzhydryl bromide in 3 batches, stir at room temperature for 3.5 hours, add water, extract with ether, wash the et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com