Method and system for producing dimethyl carbonate (DMC) by using urea two-step method
A technology of dimethyl carbonate and cycloalkylene carbonate, which is applied in the field of chemical technology, can solve the problems of many side reactions, single product structure, and low conversion rate of urea in one pass, so as to reduce equipment investment costs, avoid discharge of three wastes, The effect of high conversion rate of urea per pass
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
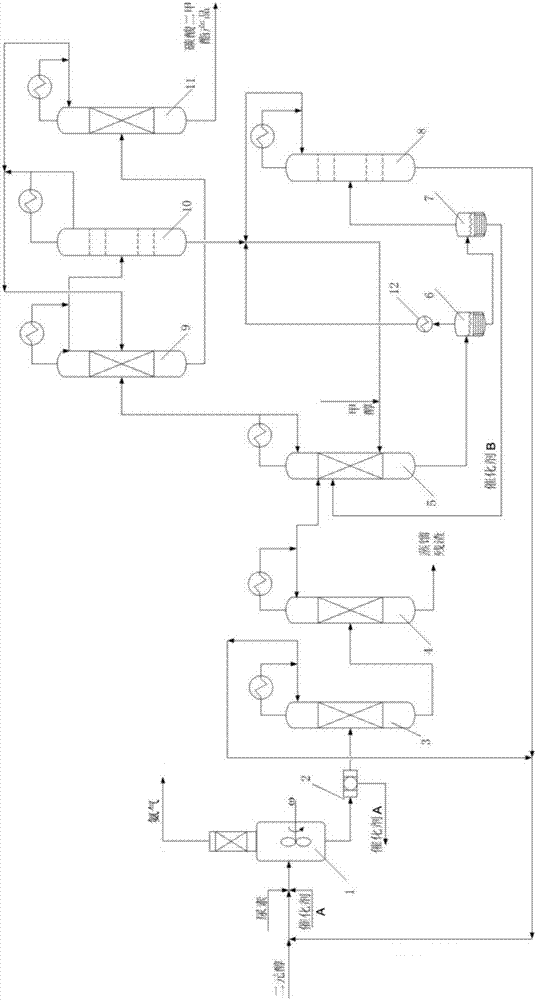
[0067] use as figure 1 The process flow shown produces dimethyl carbonate:
[0068] (1) Urea reacts with 1,2-propylene glycol to generate propylene carbonate
[0069] At first, in batching system, raw material urea and catalyst A are miscible in 1,2-propanediol, then, send into cycloalkylene carbonate synthesis reactor 1 and react, wherein, 1,2-propanediol: urea (mol Ratio)=4:1, the mass concentration of catalyst A is 2%, and catalyst A is Ni oxide supported by zinc oxide; the type of reactor 1 is full mixed flow, the total feed is 619kg / hr, and the reaction condition is 160°C , 0.102MPaA. The conversion rate of urea is 100%, the selectivity of propylene carbonate is 95%, the reaction product is 56.7kg / hr, NH 3 It is discharged from the ammonia gas recovery port and passed into the urea synthesis unit for the urea synthesis process. The reaction product propylene carbonate, together with unreacted 1,2-propanediol and catalyst A, is discharged from the bottom into the filte...
Embodiment 2
[0079] use as figure 1 The process flow shown produces dimethyl carbonate:
[0080] (1) Urea reacts with ethylene glycol to form ethylene carbonate
[0081] First, in the batching system, the raw material urea and catalyst A are miscible in ethylene glycol, and then sent to cycloalkylene carbonate synthesis reactor 1 for reaction, wherein, ethylene glycol: urea (molar ratio)=4 : 1, the mass concentration of catalyst A is 2%, and catalyst A is Ni oxide including zinc oxide support; the type of the reactor 1 is full mixed flow, the total feed is 534kg / hr, and the reaction conditions are 170°C and 0.102MPaA. The conversion rate of urea is 100%, the selectivity of ethylene carbonate is 95%, the reaction product is 56.7kg / hr, NH 3 It is discharged from the ammonia gas recovery port and passed into the urea synthesis unit for the urea synthesis process. The reaction product ethylene carbonate, together with unreacted ethylene glycol and catalyst A, is discharged from the bottom i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com