A quinoxaline azetidinone compound and its application in antitumor
A technology of azetidinone and quinoxaline, applied to quinoxaline and azetidinone compounds and their application fields in antitumor drugs, can solve problems such as unreported, achieve short synthetic route, The effect of easy availability of raw materials and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
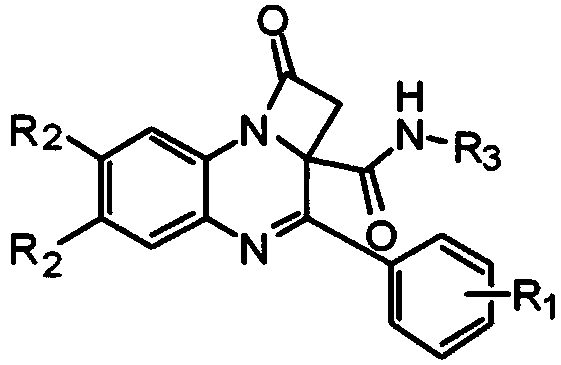
[0039] where R 1 is a hydrogen atom, R 2 is a hydrogen atom, R 3 is benzyl, that is, N-benzyl-1-oxo-3-phenyl-1,2-dihydro-2aH-azetidine[1,2-a]quinoxaline-2a-carboxamide Synthesis, the specific steps are as follows:
[0040] In a 10-mL microwave reaction tube, phenylglyoxal (1.0 mmol) and Boc-protected anthraniline (1.0 mmol) were dissolved in 2.0 mL of methanol solution, and then bromoacetic acid (1.0 mol) and benzyl isocyanide (1.0 mmol) were added to the solution in turn, the reaction solution was stirred overnight at room temperature, and then the isocyanate was detected by thin-layer chromatography. If there was no remaining isocyanine raw material, the solution was blown dry with nitrogen , and then dissolved in 5.0 ml of dimethylformamide (DMF), then added diisopropylamine (DIPA) (2.0 mmol), and reacted in a microwave oven at 90° C. for 10 minutes. The solution was diluted with ethyl acetate (15 mL), and washed three times with saturated brine, 20 mL each time. After...
Embodiment 2
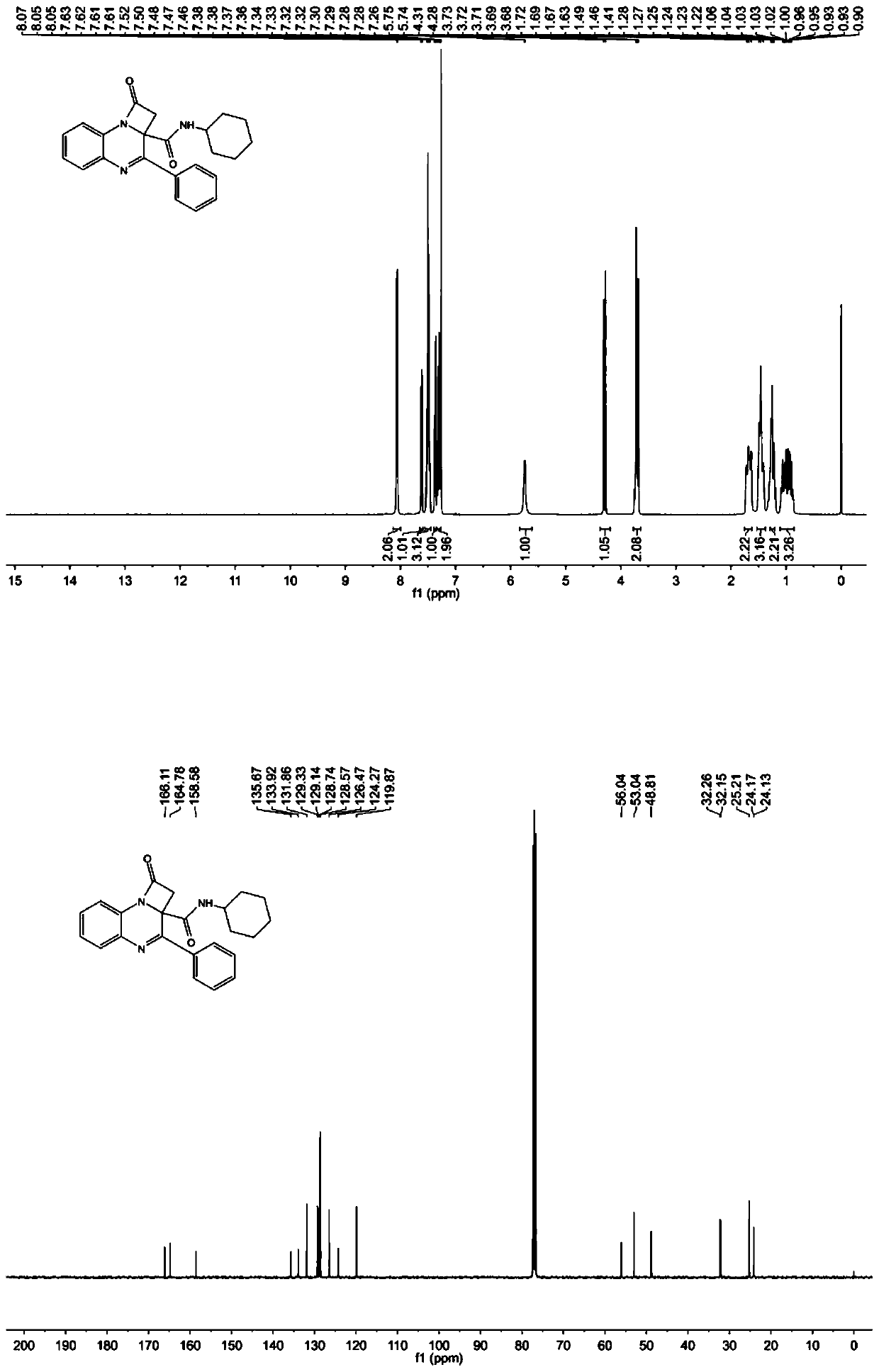
[0043] where R 1 is a hydrogen atom, R 2 is a hydrogen atom, R 3 is an alkyl group, that is, N-cyclohexyl-1-oxo-3-phenyl-1,2-dihydro-2aH-azetidine[1,2-a]quinoxaline-2a-carboxamide Synthesis, the specific steps are as follows:
[0044] In a 10-mL microwave reaction tube, phenylglyoxal (1.0 mmol) and Boc-protected anthraniline (1.0 mmol) were dissolved in 2.0 mL of methanol solution, and then bromoacetic acid (1.0 mol) and cyclohexane isocyanate (1.0 mmol) were added to the solution in turn, the reaction solution was stirred overnight at room temperature, and then the isocyanate was detected by thin-layer chromatography. If there was no remaining isocyanate raw material, the solution was blown with nitrogen dry, and then dissolved with 5.0 ml of dimethylformamide (DMF), then added diisopropylamine (DIPA) (2.0 mmol), and reacted in a microwave oven at 90° C. for 10 minutes. The solution was diluted with ethyl acetate (15 mL), and washed three times with saturated brine, 20 mL...
Embodiment 3
[0047] where R 1 is methoxy, R 2 is a hydrogen atom, R 3 For benzyl, namely N-benzyl-3-(4-methoxyphenyl)-1-oxo-1,2-dihydro-2aH-azetidine[1,2-a]quinoxa The synthesis of phenoline-2a-carboxamide, concrete steps are as follows:
[0048] In a 10 mL microwave reaction tube, first dissolve p-methoxybenzaldehyde (1.0 mmol) and Boc-protected anthraniline (1.0 mmol) in 2.0 mL of methanol solution, and then add bromine Acetic acid (1.0 mmol) and benzyl isocyanate (1.0 mmol) were successively added to the solution, the reaction solution was stirred overnight at room temperature, and then thin-layer chromatography was used to detect the isocyanate. If there was no remaining isocyanate raw material, the solution Blow dry with nitrogen, then dissolve with 5.0 ml of dimethylformamide (DMF), add diisopropylamine (DIPA) (2.0 mmol), and react in a microwave oven at 90° C. for 10 minutes. The solution was diluted with ethyl acetate (15 mL), and washed three times with saturated brine, 20 mL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com