Fluorescence probe and synthetic method thereof and application of fluorescence probe in circulating tumor cell detection
A technology for fluorescent probes and tumor cells, applied in fluorescence/phosphorescence, measuring devices, and material analysis through optical means, can solve problems such as complex operations, high costs, and poor accuracy, and achieve improved sensitivity, short time consumption, and The effect of low instrument requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Synthesis of a 2-fold amplified fluorescent probe using the Tokyo Green fluorophore as an example
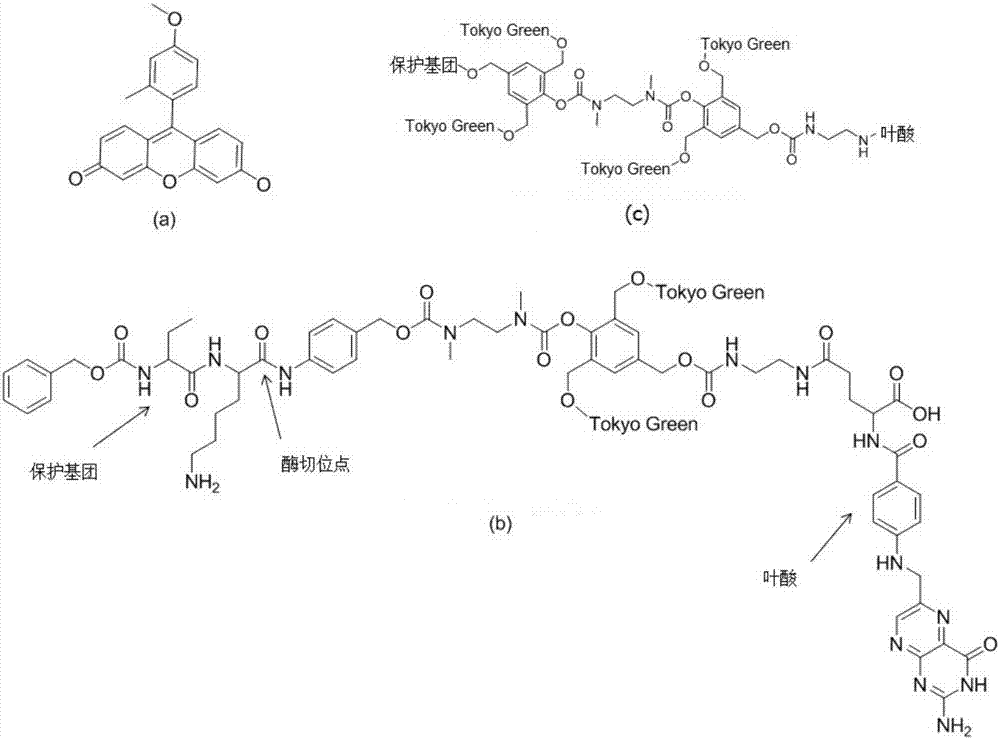
[0039]The double-amplified fluorescent probe mainly includes four parts: a phenylalanine-lysine protecting group, a self-degradation bond, a fluorescent group Tokyo Green, and a folic acid molecule that targets CTC.
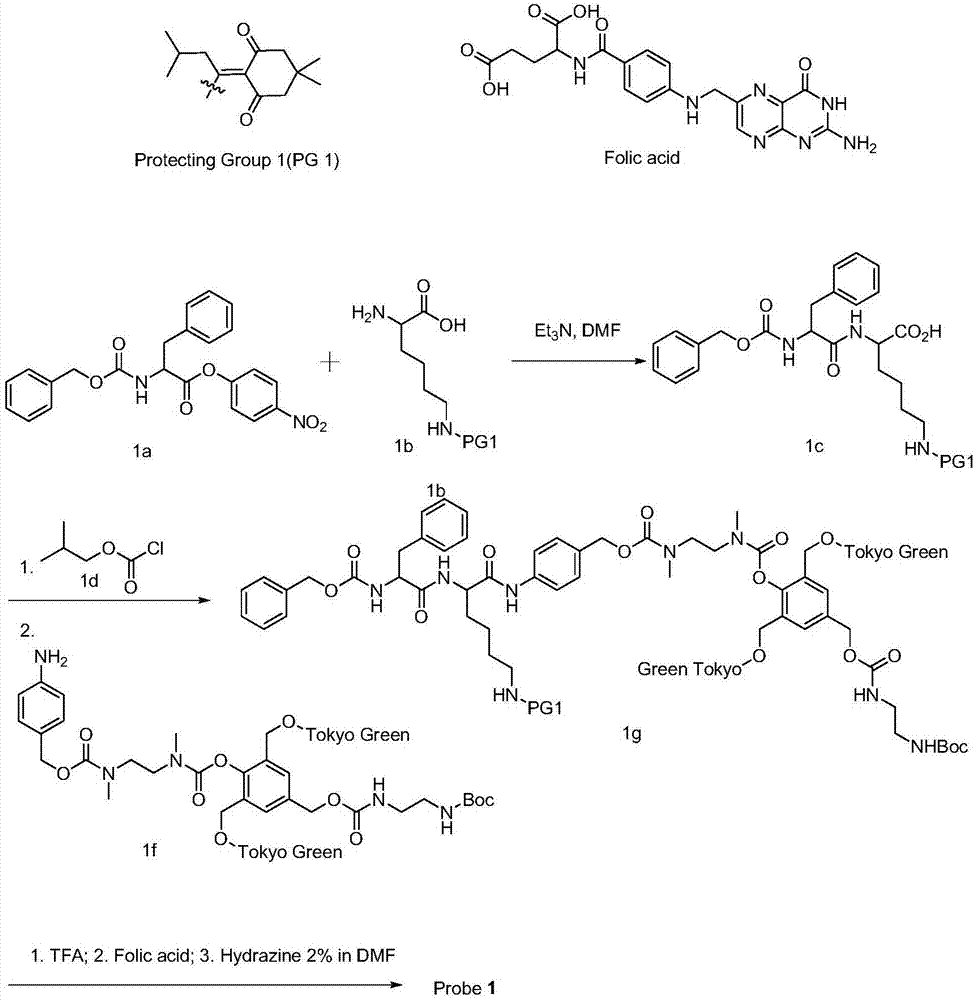
[0040] Firstly, phenylalanine ester (1a) protected by benzyloxycarbonyl reacts with lysine (1b) protected by di-tert-butyl dicarbonate (PG1) to obtain product 1c lysyl phenylalanine ester. 1d diformyl chloride
[0041] 1c reacts with diacid chloride to form an acid anhydride intermediate, and then reacts with 1f fluorescent group-lysyl phenylalanine ester to obtain intermediate 1g with two fluorescent groups connected to Tokyo Green.
[0042] Use trifluoroacetic acid to remove the Boc protecting group in compound 1g, and then react with folic acid in an amidating reagent such as EDCI / HOBt system to form 1g of a product coupled with folic acid, whi...
Embodiment 2
[0043] Example 2 CTC detection
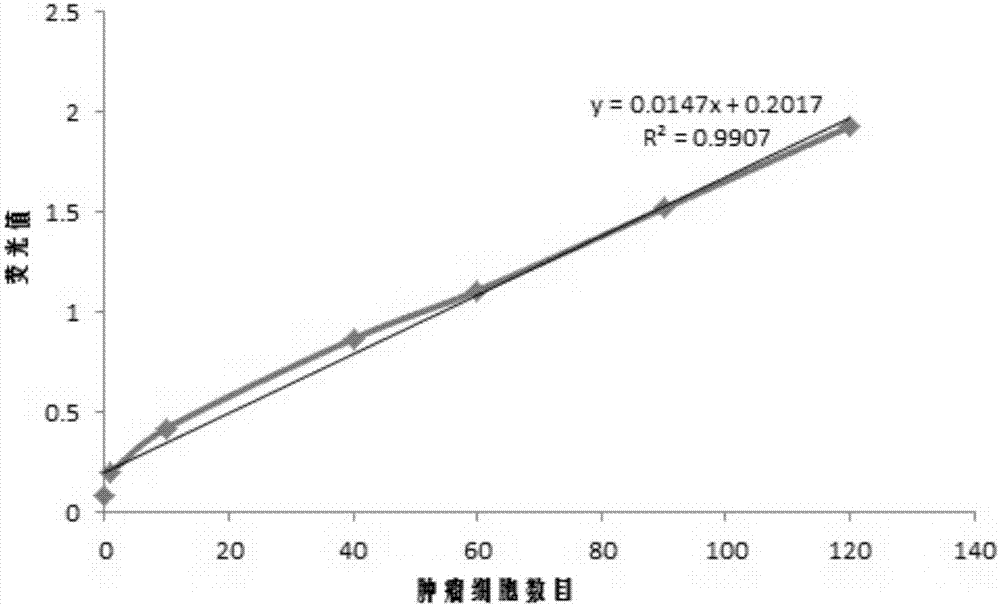
[0044] Take 8 tubes of 5mL normal human anticoagulant blood, add different numbers of colon cancer cells and mix well, discard the supernatant after centrifuging at 1500rpm for 5min, add 1mL buffer solution containing 0.1% fluorescent probe prepared in Example 1, and warm at 37°C. After incubation for 10-20min, detect with a fluorescence spectrophotometer, the results are as follows: image 3 shown.
Embodiment 3
[0045] Example 3 Mouse peritoneal tumor development
[0046] Take 0.5 mL of the buffer solution containing 0.1% fluorescent probe prepared in Example 1, inject it into the abdominal cavity of the mouse, anesthetize the mouse with barbiturate 20 minutes later, fix the limbs, cut the abdominal cavity with ophthalmic scissors, and use 488 excitation light Observe the tumor under the LED light ( Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com