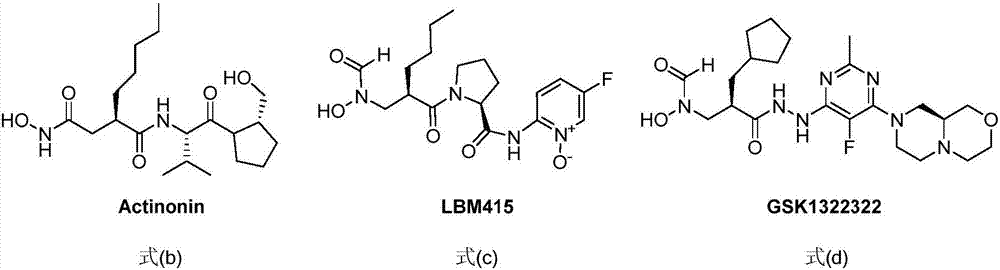
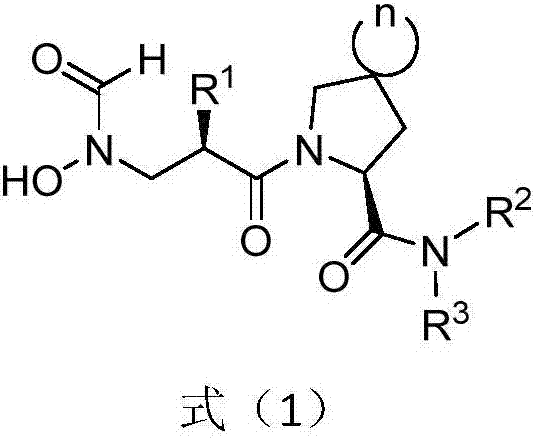
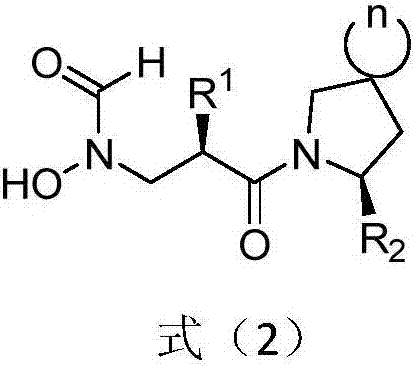
Anti-tumor application of spiro-three-membered ring and spiro-five-membered ring type peptide deformylase inhibitor
A technology of peptide deformylase and three-membered ring, which is applied in antineoplastic drugs, antibacterial drugs, and medical preparations containing active ingredients, etc., and can solve problems such as clinical manifestations, body toxicity, and metabolic instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] (S)-5-((R)-2-((N-hydroxycarboxamido)methyl)caproylamido)-N-(1H-pyrazol-3-yl)-5-azaspiro[2.4 ]Synthesis of heptane-6-amide
[0123]
[0124] Step 1: Add 3-aminopyrazole (1.50g, 18.0mmol), trimethylamine (4.5g, 20.6mmol), 4-(dimethylamino)pyridine (0.15g, 1.2mmol) in 60mL dioxane, Add Boc after stirring to dissolve 2 O, heated to reflux for 8h, after the reaction was completed, the solvent was spinned out, then diluted with EA and extracted, washed successively with 10% citric acid and saturated brine, and the organic phase was concentrated to obtain an oil, which was passed through the column (PE / DCM=2 / 1) The product was obtained as a white solid (1.6 g, yield 48%).
[0125]Step 2: the operation is as in step 1 in the synthetic general formula (X1). After adding 20mL of DMF to the acid (2.05g, 8.5mmol) to dissolve, add N-methylimidazole (1.54g, 18.7mmol) under ice cooling, then slowly add MsCl (1.07g, 9.4mmol), stir for 15min and then add Boc Protected amine (1.5...
Embodiment 2
[0136] 5-Fluoro-2-((S)-5-((R)-2-((N-hydroxycarboxamido)methyl)hexylcarbonyl)-5-azaspiro[2.4]heptane-6-amide base) synthesis of pyridine N-oxygen compounds
[0137]
[0138] Step 1: The operation is as in Step 1 in the synthesis of general formula (X1).
[0139] Step 2: The operation is as in step 2 in the synthesis of general formula (X1) (2.1 g, white solid, two-step yield 68%).
[0140] Step 3: The operation is as in step 3 in the synthesis of general formula (X2).
[0141] Step 4: The obtained oil (242mg, 0.46mmol) was dissolved in ethyl acetate (3mL), and hydrogen peroxide urea complex (133mg, 1.40mmol) and phthalic anhydride (207mg, 1.40mmol) were added. The mixture was stirred at room temperature for 2 hours. After the reaction was completed, the reaction was quenched with sodium thiosulfate and extracted with ethyl acetate. The organic phase was dried and concentrated to obtain the crude product.
[0142] Step 5: The operation is as in step 4 in the synthesis of...
Embodiment 3
[0148] (S)-N-(5-(tert-butyl)isoxazol-3-yl)-5-((R)-2-((N-hydroxycarboxamido)methyl)hexylcarbonyl)-5- Synthesis of Azaspiro[2.4]heptane-6-amide
[0149]
[0150] Step 1: The operation is as in Step 1 in the synthesis of general formula (X1).
[0151] Step 2: The operation is as in step 2 in the synthesis of general formula (X1) (1.4 g, white solid, two-step yield 47%).
[0152] 1 H NMR (400MHz,D 2 O)δ6.14(s,1H),4.44-4.41(m,1H),3.12-2.93(m,2H),2.19(dd,J=13.4,8.9Hz,1H),1.86(dd,J=13.4 ,6.1Hz,1H),0.52-0.29(m,4H).
[0153] 13 C NMR (101MHz, D 2 O) δ183.22, 167.90, 157.06, 93.24, 60.23, 52.67, 37.13, 32.51, 27.67, 20.09, 9.86, 8.49.
[0154] Step 3: The operation is as in step 3 in the synthesis of general formula (X2).
[0155] Step 4: The operation is as in step 4 in the synthesis of general formula (X2), column chromatography (DCM:MeOH=10:1) gives a white solid, and the two-step yield is 28%.
[0156] LC-MS(ESI):[M+1] + =435.24,t R =2.25min.
[0157] 1 H NMR (400MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com