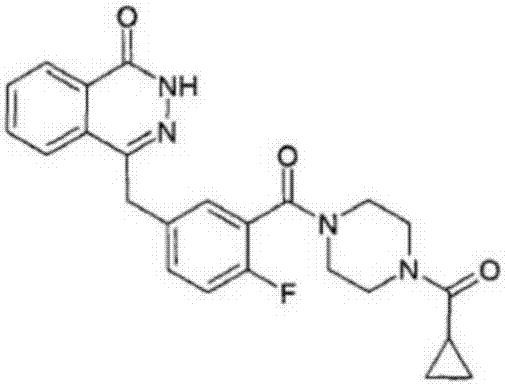
Olaparib composition capsules
A technology of composition and capsule, applied in the field of olaparib composition capsule, which can solve the problems of no further prompting, difficulty in quality control, aging, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The preparation method of olaparib capsules:
[0036] The pharmaceutical ingredients olaparib and mannitol were crushed and sieved to 100 meshes, and then the pulverized materials, dextrin, potassium metaphosphate, polacrilin potassium, and acetylated monoglyceride were mixed evenly in a mixer, and sent into Granulated in a dry granulator, 18 mesh whole granules, filled in capsules.
[0037] experiment method:
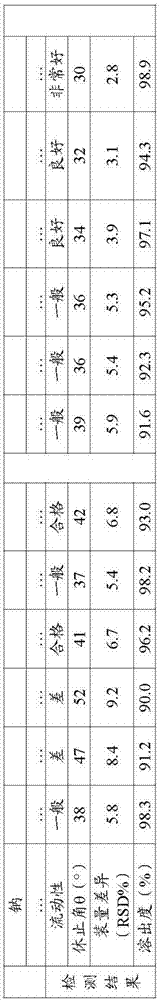
[0038] 1. Liquidity test:
[0039] The fluidity of a solid cannot be expressed by a single characteristic value, and it is often expressed by the angle of repose. It usually refers to the largest angle formed by the free slope of the powder accumulation layer and the horizontal plane. The smaller the angle of repose, the smaller the friction, and the better the fluidity. It is generally believed that when θ≤30 degrees, the fluidity is good, and when θ≤40 degrees, it can meet the fluidity requirements in the production process. The fluidity of powder has a gr...
experiment example 1
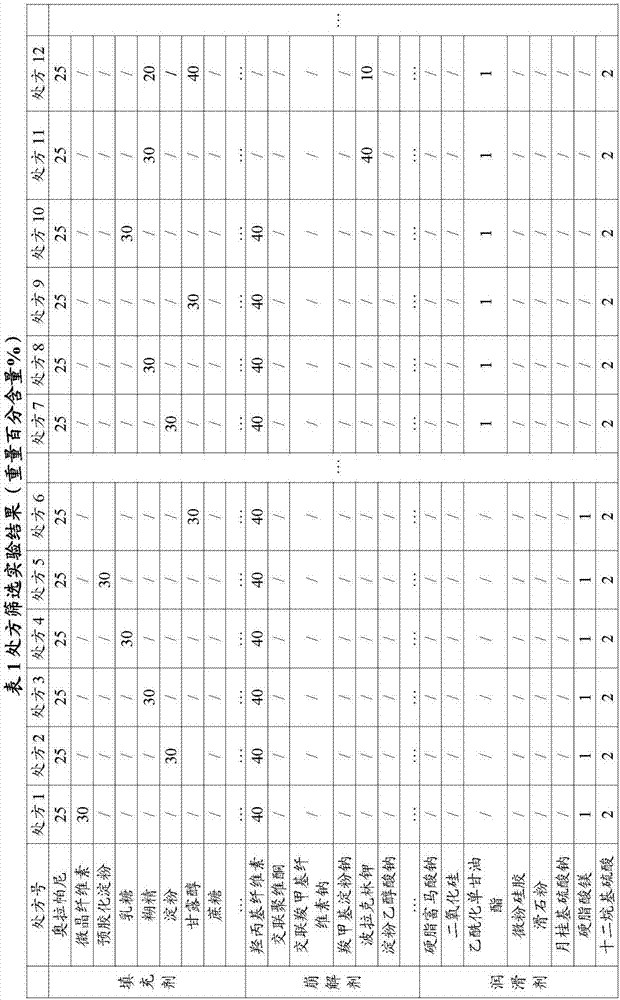
[0045] Experimental Example 1: Prescription Screening Experiment
[0046]
[0047]
[0048] Because olaparib is easy to absorb moisture and is unstable to heat and humidity, the dry granulation method is preferred to prevent the influence of the temperature of the water agent on the raw and auxiliary materials. The specific preparation method is as follows: the pharmaceutical ingredients olaparib and mannitol are pulverized Sieve 100 mesh, then put crushed material and dextrin, potassium metaphosphate, polacrilin potassium, acetylated monoglyceride in a mixer and mix evenly, send it to dry granulator for granulation, 18 mesh granulation, Capsule filling.
[0049] There are too many screening experimental data, and only some important experimental data are listed here. After a large number of experimental screenings, the inventor found that when hydroxypropyl cellulose was combined with starch, mannitol, and dextrin, the dissolution rate was not good, especially the hydro...
experiment example 2
[0050] Experimental Example 2: Screening experiment of dosage of acetylated monoglyceride and polacrilin potassium
[0051] This experimental example is when preparing olaparib capsules, as the dosage screening experiment of acetylated monoglyceride and polacrilin potassium, the weight percentage content of each raw material is controlled: olaparib 23%, mannitol 40%, 25% dextrin and 0.5% potassium metaphosphate, on the basis of which the weight percent content of acetylated monoglyceride and polacrilin potassium is adjusted.
[0052] The dosage of disintegrant polacrilin potassium has great influence on the disintegration time and dissolution rate, and its dosage is too small to meet the requirements of disintegration and dissolution. The greater the dosage of polacrilin potassium, the slower the disintegration and dissolution rate. Therefore, the inventors selected 8.5%-10.5% by weight after a large number of experimental screenings.
[0053]
[0054] The above-mentioned...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com