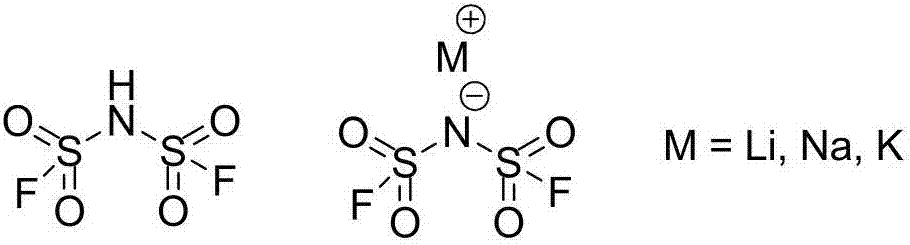
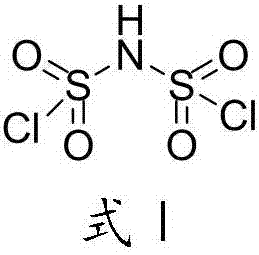
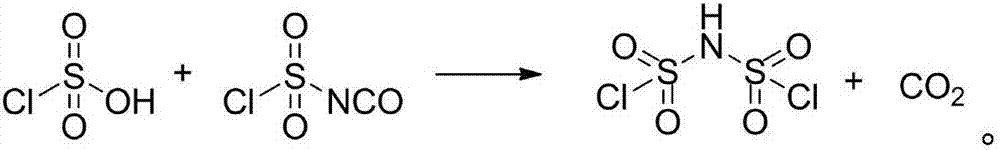
Difluoro-sulfonyl imine preparation method and method for preparing difluoro-sulfonyl imine alkali metal salt by using prepared difluoro-sulfonyl imine
A technology of bisfluorosulfonimide base and bisfluorosulfonimide, which is applied in the field of fluorine chemical industry, can solve the problem of hindering the large-scale application of bisfluorosulfonimide and its alkali metal salts, mainstream electrolyte materials, and production processes Long process and other issues, to achieve the effect of reducing material consumption and waste generation, simple reaction equipment, and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of polystyrene-supported antimony pentafluoride, bischlorosulfonimide and bisfluorosulfonimide, and activation of polystyrene-supported antimony pentafluoride
[0033] After 100g of polystyrene was dried under vacuum at 60°C for 6h, it was added to 1000g of dichloromethane to swell for 4h, then 400g of antimony pentafluoride was added, and the reaction was stirred at 30°C. The polystyrene microspheres gradually turned red and ended after 12h. reaction. Suction filtration, washing with dichloromethane, toluene and acetone respectively, and vacuum drying to obtain 400 g gray polystyrene antimony pentafluoride microspheres.
[0034] Under agitation, add 580g chlorosulfonic acid (5mol) and 721g chlorosulfonyl isocyanate successively in a dry 2L reaction vessel, then heat the mixed solution to 120°C for reaction, and use a carbon dioxide gas detector to detect that the carbon dioxide concentration is less than 500ppm. The reaction was stopped, and the ...
Embodiment 2
[0038]Example 2: Preparation of lithium bisfluorosulfonyl imide Under stirring, 580 g of chlorosulfonic acid (5 mol) and 721 g of chlorosulfonyl isocyanate were successively added to a dry 2L reaction vessel, and then the mixture was heated to 120°C for reaction, using After the carbon dioxide gas detector detects that the carbon dioxide concentration is less than 500ppm, stop the reaction, carry out vacuum distillation to the obtained brownish yellow liquid crude product, collect the cut of 80-85 ℃ / 30Pa, obtain 856g (yield 80%) dichlorosulfonyl Amine is a colorless liquid.
[0039] Under constant temperature stirring at 25°C, add 400g of polystyrene-loaded antimony pentafluoride, 600g of acetonitrile, and 42.4g of anhydrous lithium chloride into a 2L three-neck flask, and then slowly add 214g of dichlorosulfonimide (1mol) for fluorination Reaction, after adding dichlorosulfonimide dropwise, keep the constant temperature of 25°C to continue the reaction for 6 hours, and then f...
Embodiment 3
[0040] The preparation of embodiment 3 bisfluorosulfonimide lithium
[0041] Under constant temperature stirring at 25°C, add 400g of polystyrene-loaded antimony pentafluoride and 600g of acetonitrile into a 2L three-neck flask, then slowly add 214g of dichlorosulfonimide (1mol) for fluorination reaction, and dropwise add dichlorosulfonimide After the imide, add 42.4g of anhydrous lithium chloride to the system, keep a constant temperature of 25°C to continue the reaction for 6 hours, and then filter with suction. The filter cake is polystyrene-supported antimony pentafluoride, which is used after activation, and the filtrate is rotary evaporated After removing the solvent, 160 g (yield 85.6%) of lithium bisfluorosulfonyl imide remained. The obtained crude lithium bisfluorosulfonyl imide was recrystallized using a mixed solvent of acetonitrile and dichloromethane at a mass ratio of 1:1 to obtain 146 g of a white powdery solid with a yield of 78.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com