Preparation method and application of controllable CD20 chimeric antigen receptor modified T cell
A chimeric antigen receptor and cell technology, which is applied in the field of preparation of controllable CD20 chimeric antigen receptor modified T cells, can solve the problems of reducing expression and restriction, and achieve the effect of fast proliferation and broad tumor killing spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The construction of embodiment 1pLent-C-GFP-CD20 expression vector
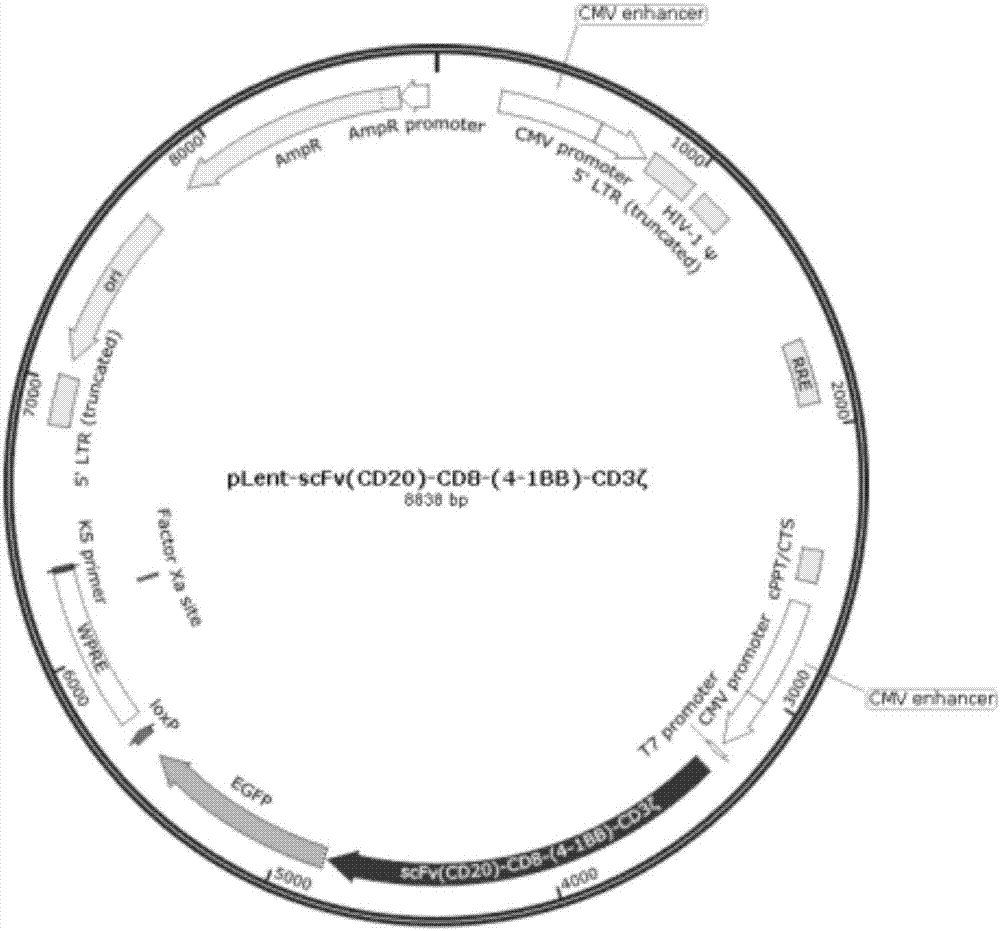
[0026] Leader-CD20-CD8-4-1BB-CD3ζ-T2A-HSV-TK module opinion figure 1 (See appendix SEQ ID NO.1 for the complete nucleic acid sequence).
[0027] Each module sequence of Leader-CD20-CD8-4-1BB-CD3ζ-T2A-HSV-TK
[0028] (1) Leader (SEQ ID NO.2)
[0029] (2) Humanized anti-human B-cell lymphoma CD20 single-chain antibody (SEQ ID NO.3)
[0030] (3) CD8Hinge region (SEQ ID NO.4)
[0031] (4) CD8 transmembrane region (SEQ ID NO.5)
[0032] (5) 4-1BB intracellular region (SEQ ID NO.6)
[0033] (6) CD3ζ intracellular region (SEQ ID NO.7)
[0034] (7) Self-cleaving polypeptide T2A (SEQ ID NO.8)
[0035] (8) HSV-TK sequence (SEQ ID NO.9)
[0036] According to the above sequence from (1)-(8), entrust Beijing Biomed Gene Technology Co., Ltd. to synthesize its entire expression cassette, and insert it into the pLent-C-GFP vector (purchased from Vigene Company) AsiSI-NotI site (see figure 2 ), transformed int...
Embodiment 2
[0037] Example 2 Preparation of pLent-C-GFP-CD20 modified T cells
[0038] 1. Preparation of heterogeneous T cells
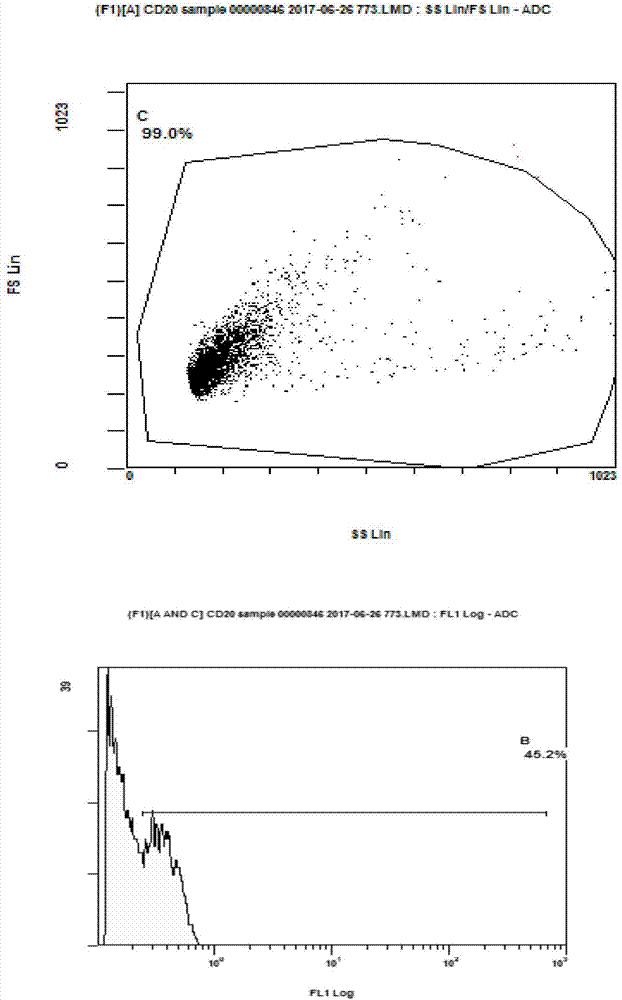
[0039] Take 75ml of autologous peripheral blood from the patient, and separate peripheral blood mononuclear cells with TBD sample density separation medium (purchased from Tianjin Haoyang Huake Biology). After inducing culture for 24 hours with a medium (purchased from CORNING Company, 88-551-CM) containing 1000 IU / ml of recombinant interferon α2a (purchased from Shenyang Sansheng Pharmaceutical), 1500 IU / ml of recombinant interleukin 2 ( purchased from Shenyang Sansheng Pharmaceutical), 50ng / ml of OKT-3 and 5% of the patient's autologous plasma to induce further culture. Doubling liquid was added every three days, cultured until the 14th day, and the positive expression rate of CD3+ and CD56+ in T cells was detected by flow cytometry (CD3-FITC, CD16 / CD56-PE antibodies were purchased from BECKMAN, A07735). CD3+ positive rate>80%, CD3+CD56+ double positive rate...
Embodiment 3
[0044] Example 3: Research on the killing activity of CAR-T cells with a suicide gene system
[0045] The human B lymphoma cell line RAMOS was used as target cells, and the effector cells were CAR-T cells and T cells infected with empty lentivirus.
[0046] 1. Control of CAR-T cell activity by suicide gene system
[0047] The CAR-T cells were divided into 6×10 4 cells / ml inoculated in 96-well plate, 100ul per well, placed in 5% CO 2 , Cultivate in a 37°C incubator for 24 hours; add 10 ng of ganciclovir (Wuhan Haite Biopharmaceutical Co., Ltd.), once every 24 hours, a total of 3 times. CAR-T cells without ganciclovir were used as negative control. Detect the amount of remaining cells in the 96-well plate by the MTT method, the cell survival rate=(experimental group OD-blank group OD) / (control group OD-blank group OD), the control rate of the suicide gene system to the modified T cell activity=( 1-cell viability)×100%. The control rate of the suicide gene system on the acti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com