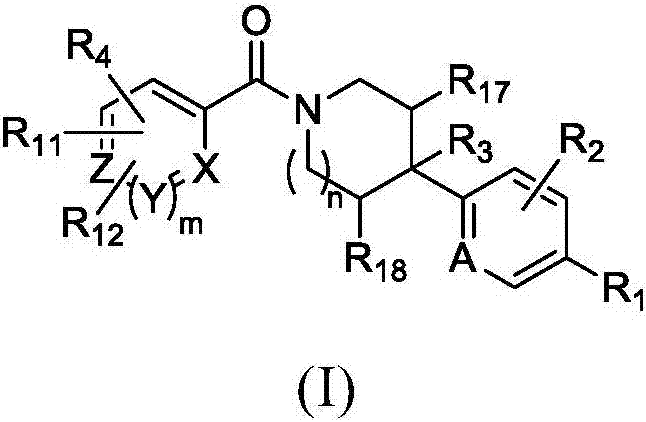
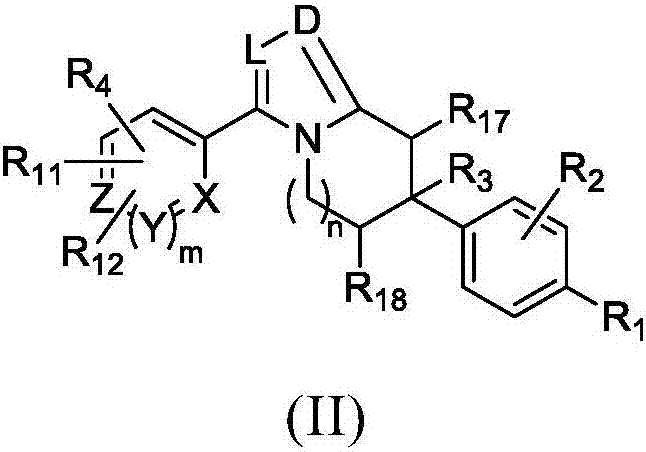
Heterocyclic modulators of lipid synthesis
A compound, cycloalkyl technology, applied in the field of heterocyclic regulators of lipid synthesis, which can solve the problems of side effects, hematopoietic and intestinal epithelial cell destruction, lack of improvement in cancer survival rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1289] Example 1 - FASN Inhibition by Compounds of the Disclosure
[1290] Determination of FASN biochemical activity: FASN enzyme was isolated from SKBr3 cells. SKBr3 is a human breast cancer cell line with high levels of FASN expression. It is estimated that FASN comprises approximately 25% of the cytosolic protein in this cell line. SKBr3 cells were homogenized in a Dunes homogenizer and then centrifuged at 4°C for 15 min to remove particulate matter. Supernatants were then analyzed for protein content, diluted to appropriate concentrations, and used to measure FASN activity. The presence of FASN was confirmed by Western blot analysis. A similar method of isolating FASN from SKBr3 cells is described in Teresa, P. et al. (Clin. Cancer Res., 2009; 15(24), 7608-7615).
[1291] FASN activity of SKBr3 cell extracts was determined by measuring NADPH oxidation or the amount of thiol-containing coenzyme A (CoA) released during fatty acid synthase reactions. The dye CPM (7-di...
Embodiment 2
[1292] Example 2 - Antiviral Activity
[1293] Antiviral activity of Formulas (I-Z) was assessed using the HCV1b replicon system:
[1294]
[1295]The ET (luc-ubi-neo / ET) cell line, the Huh7 human hepatoma cell line carrying a stable luciferase (Luc) reporter gene and three cell culture-adaptive mutations in the HCV replicon, was used to construct the replicon (Pietschmann et al. , (2002) "Journal of Virology (J. Virol.), 76:4008-4021). The HCV Replicon Antiviral Evaluation Assay investigated the effect of compounds at six half-log concentrations. Human interferon alpha-2b was included as a positive control compound in each run. Subconfluent cultures of the ET line were seeded into 96-well plates dedicated to analysis of cell number (cytotoxicity) or antiviral activity, and the next day drugs were added to the appropriate wells. Cells were processed 72 hours later when they were still subconfluent. Determination of EC 50 (Concentrations that inhibit the replicon by 5...
Embodiment 3
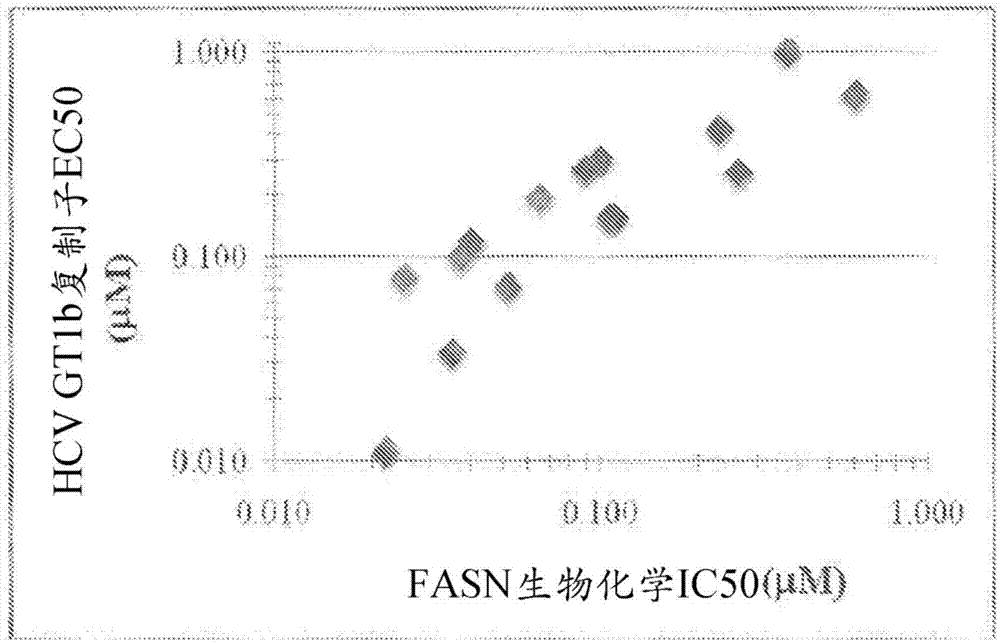
[1298] Example 3 - FASN Inhibition Correlates with HCV Inhibition
[1299] The antiviral activity of 15 compounds of the present disclosure (numbered in relation to the compounds in Table 1) was measured using the HCV replicon system. According to published method (Lohmann et al., (1999) " Science (Science) ", 285 (5424): 110-113; Lohmann et al., (2001) " Journal of Virology (J.Virol.) ", 75 (3): 1437-1449; and Qi et al., (2009) Antiviral Res., 81(2):166-173), using Huh7 selected by G418, to establish the replicon cell line 1b (HCV1b / Luc- Neo replicon (1b Con1 with firefly gene integrated)). Replicon assembly using synthetic gene fragments. The GT1b line has PV-EKT and carries 3 adaptive mutations E1202G(NS3), T1280I(NS3), K1846T(NS4B), and the backbone is Con1. The medium is:
[1300] a) DMEM supplemented with 10% FBS, G418 (250 μg / ml), streptomycin (100 μg / ml) / penicillin (100 U / ml), L-glutamine (100×), NEAA (100×)
[1301] b) Medium prepared as follows:
[1302] i) 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com