Group-I type-4 aviadenovirus DNA vaccine, and preparation method and application thereof
A DNA vaccine and avian adenovirus technology, applied in the field of DNA vaccines, can solve unseen problems and achieve the effects of low cost, convenient use and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
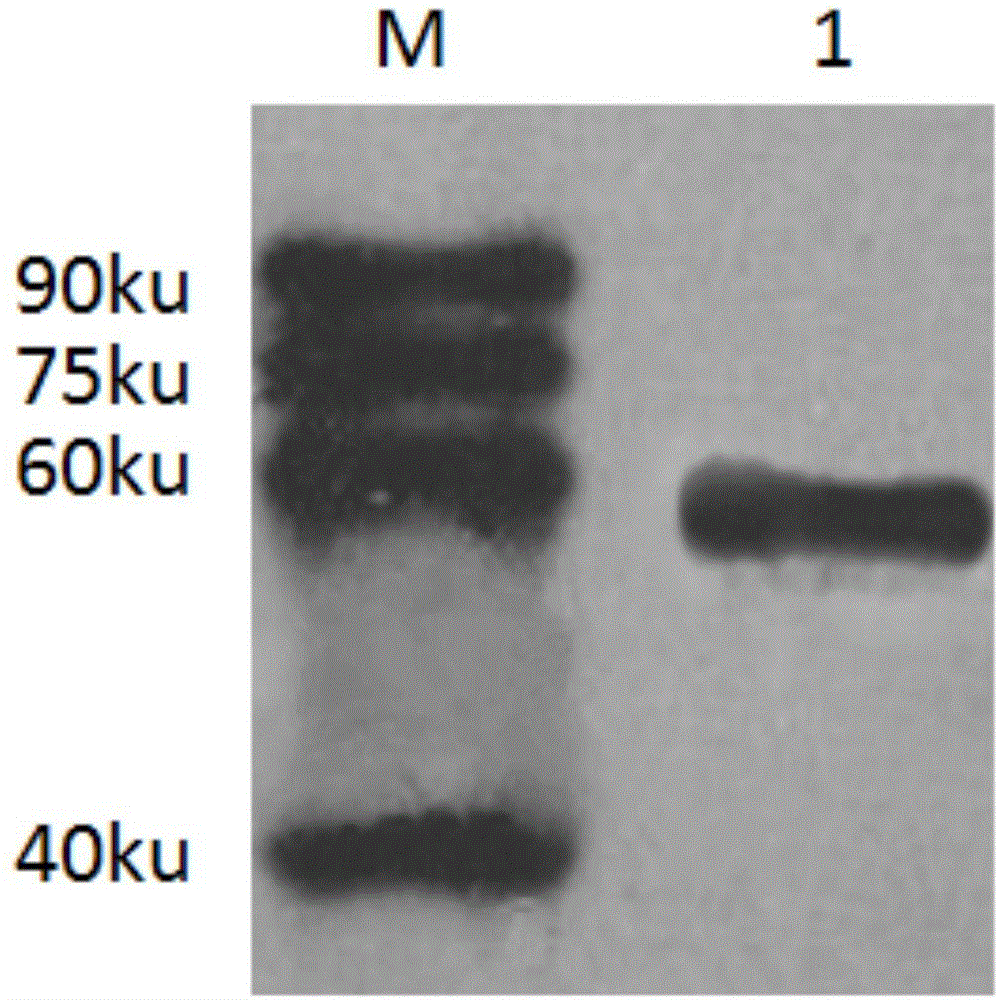
Image
Examples
Embodiment 1
[0033] A kind of I group 4 type avian adenovirus DNA vaccine of the present embodiment contains I group 4 type avian adenovirus penton gene, the sequence of the I group 4 type avian adenovirus penton gene is shown in SEQ ID No.1 Show.
Embodiment 2
[0035] The preparation method of a kind of I group 4 type poultry adenovirus DNA vaccine of the present embodiment comprises the following steps:
[0036] (1) Proliferation of group I adenovirus type 4
[0037] The virulent strain of avian adenovirus type 4 of Group I was isolated from diseased broilers; the virus was inoculated on LMH cells grown into a single layer, adsorbed for 1 hour at 37°C, added DMEM maintenance solution with 5% serum concentration, and continued to cultivate until 60- After 64 hours, when the cells appear round shrinkage, detachment and other lesions, collect the poison and store at -70°C for later use;
[0038] (2) Primer design and synthesis
[0039]①According to the genome sequence of group I group 4 avian adenovirus (Gene ID: 10399473), select the penton gene (penton) sequence, and use Oligo7 software to design a pair of specific primers for the penton gene:
[0040] Primer 1: 5'-CGGAATTCATGTGGGGGTTGCAGCCG-3',
[0041] Primer 2: 5'-GTCGACGTCGCGG...
Embodiment 3
[0053] The safety and immune effect detection of a kind of I group type 4 avian adenovirus DNA vaccine of the present embodiment comprises the following steps:
[0054] (1) Vaccine safety testing
[0055] Use 20 3-week-old SPF chickens, 10 chickens in the first group, inject 200 μg of the pCI-penton vaccine prepared in the above examples into the leg muscles (100 μg for each of the two legs), and 10 chickens in the second group as a control In the group, 200 μg of plasmid pCI was injected intramuscularly into the legs, and observed continuously for 2 weeks to observe whether the immunized chickens had any local and systemic adverse reactions caused by the injection of the vaccine.
[0056] The results showed that the spirit and diet of chickens in the two groups were normal after immunization, and there was no abnormal reaction such as redness and swelling at the injection site.
[0057] (2) Detection of vaccine immune effect
[0058] 20 2-week-old SPF chickens were randomly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com