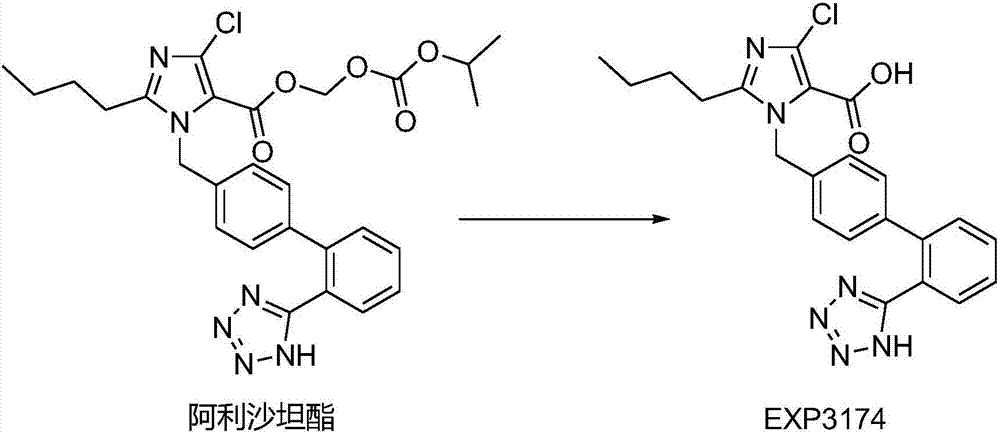
Allisartan isoproxil solid dispersion as well as preparation method thereof and preparation containing solid dispersion
A technology of alisartan medoxomil and solid dispersion, which is applied in the direction of medical preparations containing active ingredients, medical preparations with non-active ingredients, pill delivery, etc. It can solve the problems of long process time, complicated process of fluidized bed top spraying method, etc. Process complexity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
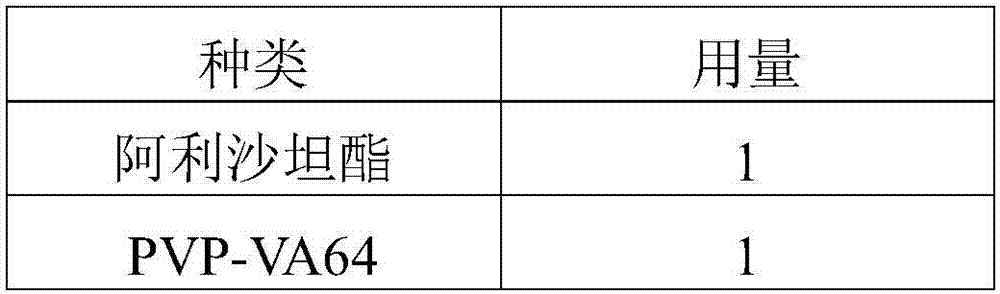
[0081] Embodiment 1 solid dispersion preparation
[0082] type
Dosage
alisartan medoxomil
1
PVP-K30
2
0.06
0.03
[0083] (1) Mix allisartan medoxomil and auxiliary materials uniformly to make a physical mixture;
[0084] (2) Set the extrusion temperature to 130°C. After the temperature stabilizes, add the physical mixture in step (1) to the hot-melt extruder at a constant speed, and extrude strip-shaped extrudates;
[0085] (3) Cool the strip-shaped extrudate obtained in step (2) and pulverize it through a 60-mesh sieve to obtain a solid dispersion of alisartan medoxomil.
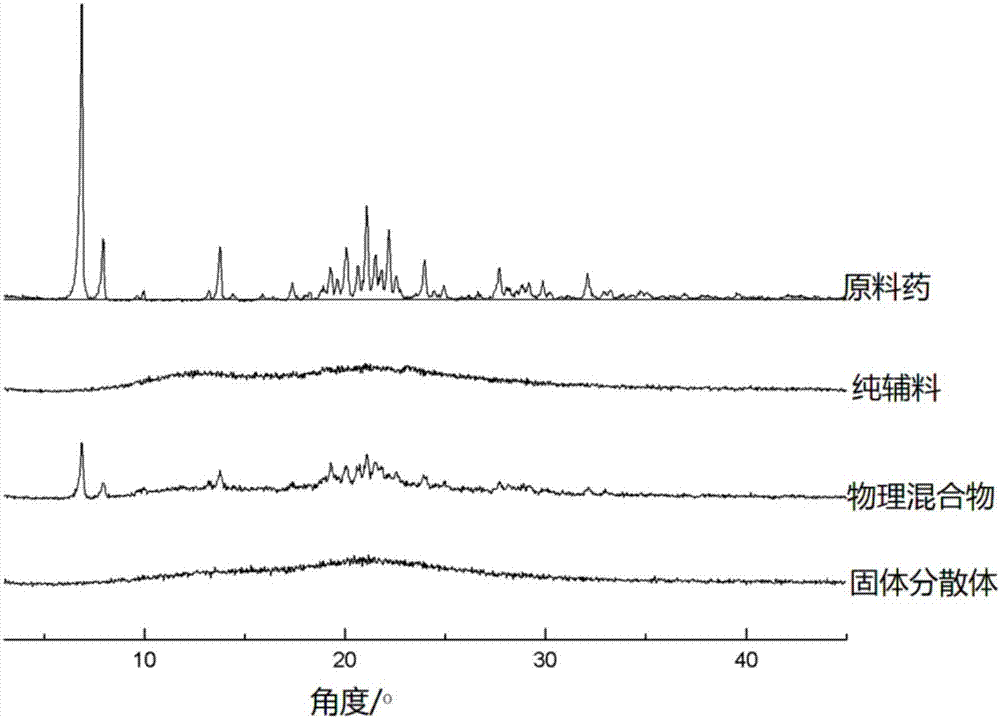
[0086] The XRD spectrogram of gained solid dispersion is compared with the XRD spectrogram of crude drug, pure adjuvant and physical mixture (contrast figure is as follows figure 1 Shown), by the comparison of the XRD powder diffraction pattern, it can be seen that Alisartan medoxomil exists in a nearly amorphous form in ...
Embodiment 2
[0087] Embodiment 2 solid dispersion preparation
[0088] type
Dosage
alisartan medoxomil
1
PVP-K30
1
0.06
0.03
[0089] (1) Mix allisartan medoxomil and auxiliary materials uniformly to make a physical mixture;
[0090] (2) Set the extrusion temperature to 115°C, and after the temperature stabilizes, add the physical mixture in step (1) into the hot-melt extruder at a constant speed, and extrude strip-shaped extrudates;
[0091] (3) Cool the strip-shaped extrudate obtained in step (2) and pulverize it through a 60-mesh sieve to obtain a solid dispersion of alisartan medoxomil.
[0092] Through detection, it is found that alisartan medoxomil exists in an approximately amorphous form in the solid dispersion, which shows that the melt extrusion process is successful in preparing the alisartan medoxomil solid dispersion.
Embodiment 3
[0093] Embodiment 3 solid dispersion preparation
[0094] type
Dosage
alisartan medoxomil
1
PVP-K30
0.8
0.7
0.06
talcum powder
0.03
[0095] (1) Alisartan medoxomil and auxiliary materials are mixed evenly to make a physical mixture;
[0096] (2) Set the extrusion temperature to 100°C, and after the temperature stabilizes, add the physical mixture in step (1) into the hot-melt extruder at a constant speed, and extrude strip-shaped extrudates;
[0097] (3) Cool the strip-shaped extrudate obtained in step (2) and pulverize it through a 60-mesh sieve to obtain a solid dispersion of alisartan medoxomil.
[0098] Through detection, it is found that alisartan medoxomil exists in an approximately amorphous form in the solid dispersion, which shows that the melt extrusion process is successful in preparing the alisartan medoxomil solid dispersion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com