Extraction and boron removal method for lithium-containing brine
An extraction and brine technology, applied in chemical instruments and methods, water pollutants, flotation water/sewage treatment, etc., can solve the problems of octanol dissolution loss and low extraction precision, and achieve the effect of increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
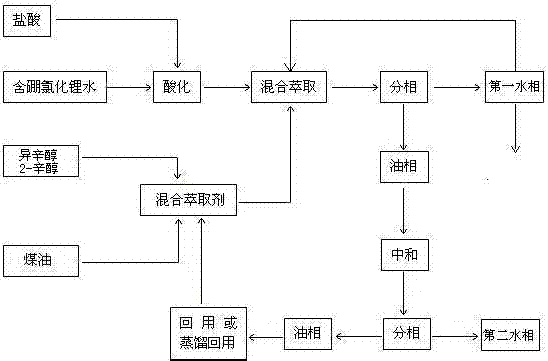
[0034] The boron-containing lithium chloride aqueous solution to be treated has a boron content of 600 ppm, and the boron-containing lithium chloride aqueous solution is acidified to pH 2 with hydrochloric acid;
[0035] Mix iso-octanol and 2-octanol in a volume ratio of 11:1 to obtain an octanol mixed solution, and mix an octanol mixed solution with kerosene in a volume ratio of 6:4; make a mixed extractant, which can be used in the environment The temperature is 25°C, after fully mixing the acidified boron-containing lithium chloride water and the mixed extractant, let it stand for 2 hours;
[0036] After standing still, the mixed liquid phase separation of boron-containing lithium chloride water and mixed extractant is an oil phase and a first water phase;
[0037] Export the first aqueous phase and detect the boron content. If the boron content exceeds the standard, it is returned to step (2) and extracted as boron-containing lithium chloride water. The operation is repeat...
Embodiment 2
[0040] The boron-containing lithium chloride aqueous solution to be treated has a boron content of 600 ppm, and the boron-containing lithium chloride aqueous solution is acidified to pH 2 with hydrochloric acid;
[0041] Mix iso-octanol and 2-octanol in a volume ratio of 13:1 to obtain octanol mixed solution, and mix octanol mixed solution with kerosene in a volume ratio of 6:4; make a mixed extractant, which can be used in the environment The temperature is 25°C, after fully mixing the acidified boron-containing lithium chloride water and the mixed extractant, let it stand for 2 hours;
[0042] After standing still, the mixed liquid phase separation of boron-containing lithium chloride water and mixed extractant is an oil phase and a first water phase;
[0043] Export the first aqueous phase and detect the boron content. If the boron content exceeds the standard, it is returned to step (2) and extracted as boron-containing lithium chloride water. The operation is repeated 3 t...
Embodiment 3
[0046] The boron-containing lithium chloride aqueous solution to be treated has a boron content of 600 ppm, and the boron-containing lithium chloride aqueous solution is acidified to pH 2 with hydrochloric acid;
[0047] Mix iso-octanol and 2-octanol in a volume ratio of 14:1 to obtain an octanol mixed solution, and mix an octanol mixed solution with kerosene in a volume ratio of 6:4; prepare a mixed extractant, which can be used in the environment The temperature is 25°C, after fully mixing the acidified boron-containing lithium chloride water and the mixed extractant, let it stand for 2 hours;
[0048] After standing still, the mixed liquid phase separation of boron-containing lithium chloride water and mixed extractant is an oil phase and a first water phase;
[0049]Export the first aqueous phase and detect the boron content. If the boron content exceeds the standard, it is returned to step (2) and extracted as boron-containing lithium chloride water. The operation is repe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com