Peripheral drug-eluting stent and its preparation and application
A technology for eluting stents and drugs, applied in the fields of medical science, surgery, coating, etc., can solve the problems of affecting the efficacy of peripheral drug-eluting stents, poor sustained release effect, etc., to prevent restenosis, prevent early burst release, strong effect of drug action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
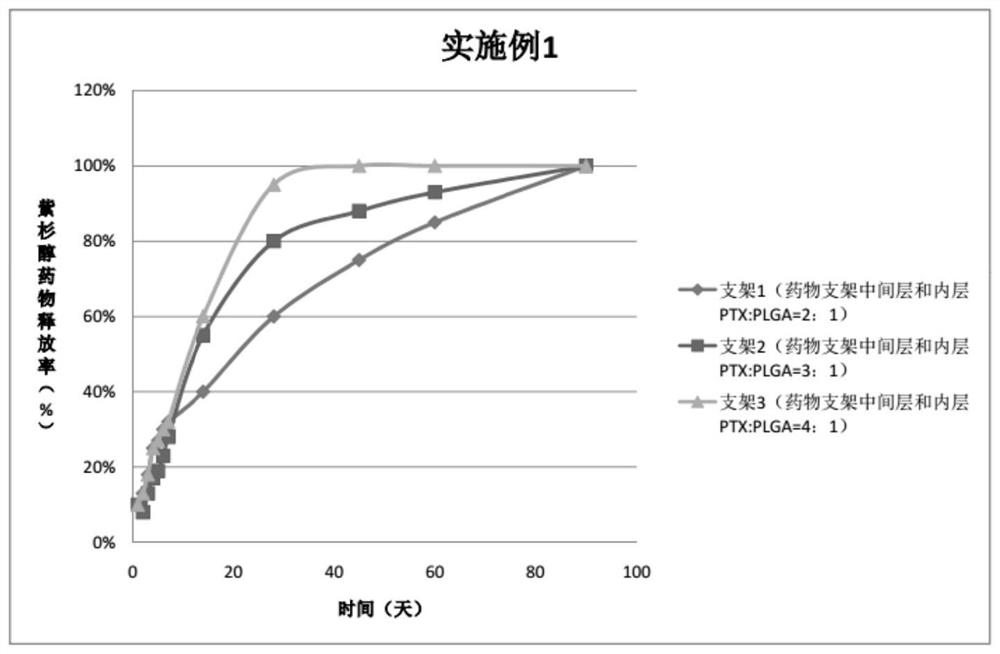
Embodiment 1
[0038] For the three peripheral drug-eluting stents provided in this embodiment, three layers of drug coatings are respectively sprayed on the outer sides of the three bare metal stents. The specific preparation of peripheral vascular stents 1, 2, and 3 is as follows:
[0039] Preparation of spray solution for stent 1:
[0040] Drug configuration for the outer layer of the drug stent: weigh 10 mg of PLGA (molecular weight 30,000, PLA:PGA=70:30), add 10 ml of tetrahydrofuran, and stir magnetically for 30-60 minutes to completely dissolve the polymer;
[0041] Drug configuration in the middle layer of the drug stent: Weigh 20mgPTX and 10mgPLGA (molecular weight 20000, PLA:PGA=50:50), add 10ml tetrahydrofuran respectively, and stir magnetically for 30-60min to completely dissolve the polymer;
[0042] Drug configuration in the inner layer of the drug stent: Weigh 20 mg of PTX and 10 mg of PLGA (molecular weight 20,000, PLA:PGA=80:20), add 10 ml of tetrahydrofuran respectively, a...
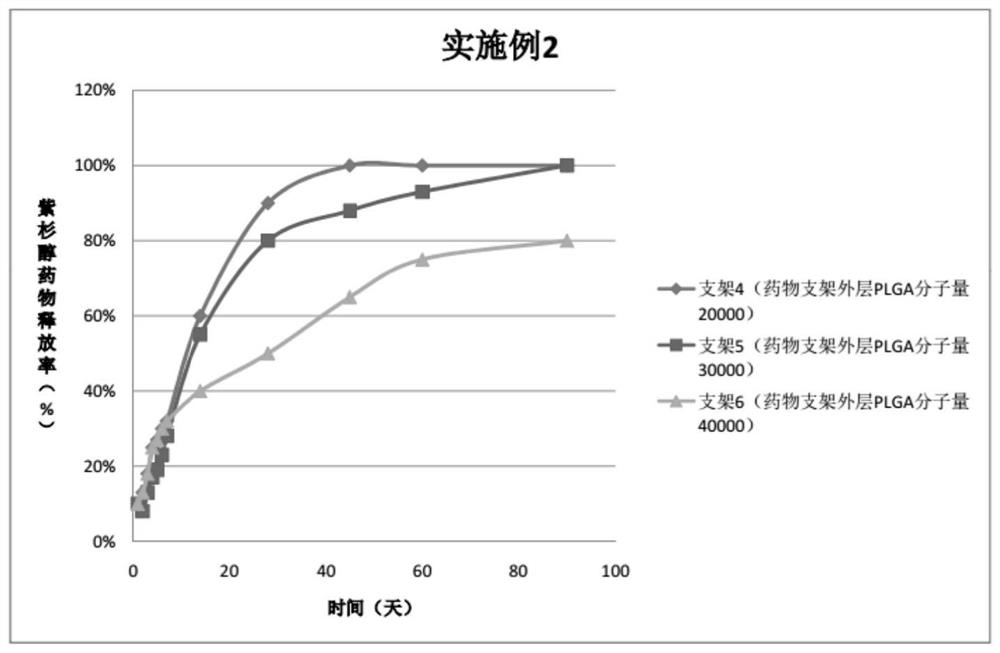
Embodiment 2
[0054] For the three peripheral drug-eluting stents provided in this embodiment, three layers of drug coatings are respectively sprayed on the outer sides of the three bare metal stents. The specific preparation of peripheral vascular stents 4, 5, 6 is as follows:
[0055] Preparation of spraying liquid medicine for bracket 4:
[0056] Drug configuration for the outer layer of the drug stent: weigh 10 mg of PLGA (molecular weight 20,000, PLA:PGA=70:30), add 10 ml of tetrahydrofuran, and stir magnetically for 30-60 minutes to completely dissolve the polymer;
[0057] Drug configuration in the middle layer of the drug stent: weigh 30mgPTX and 10mgPLGA (molecular weight 20000, PLA:PGA=50:50), add 10ml tetrahydrofuran respectively, and stir magnetically for 30-60min to completely dissolve the polymer;
[0058] Drug configuration in the inner layer of the drug stent: Weigh 30 mg of PTX and 10 mg of PLGA (molecular weight 20,000, PLA:PGA=80:20), add 10 ml of tetrahydrofuran respect...
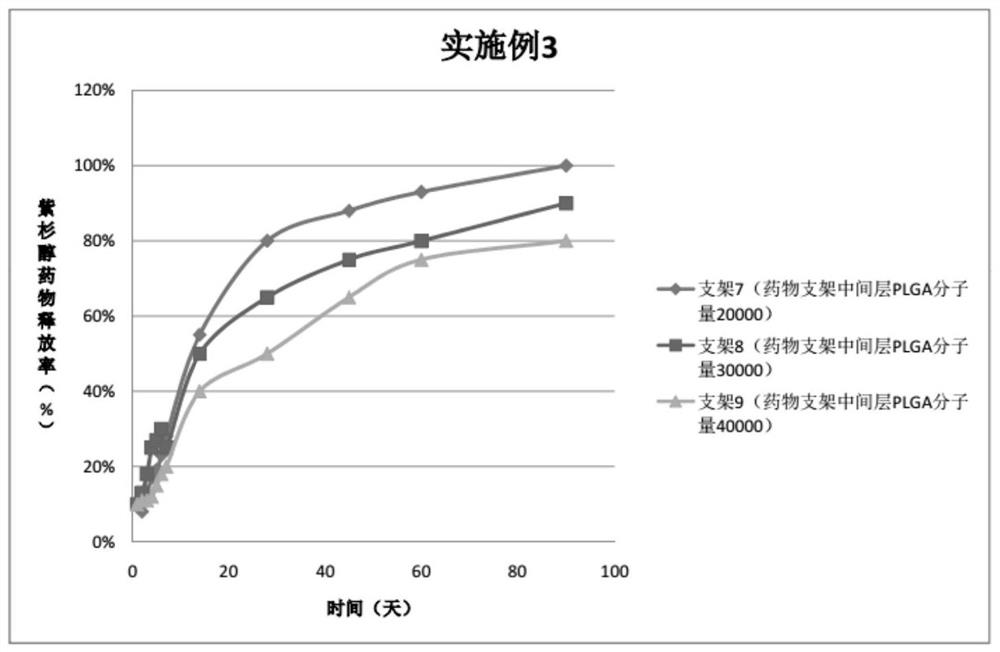
Embodiment 3
[0070] For the three peripheral drug-eluting stents provided in this embodiment, three layers of drug coatings are respectively sprayed on the outer sides of the three bare metal stents. The specific preparation of peripheral vascular stents 7, 8, 9 is as follows:
[0071] Preparation of the spraying solution of the stent 7:
[0072] Drug configuration for the outer layer of the drug stent: weigh 10 mg of PLGA (molecular weight 30,000, PLA:PGA=70:30), add 10 ml of tetrahydrofuran, and stir magnetically for 30-60 minutes to completely dissolve the polymer;
[0073] Drug configuration in the middle layer of the drug stent: weigh 30mgPTX and 10mgPLGA (molecular weight 20000, PLA:PGA=50:50), add 10ml tetrahydrofuran respectively, and stir magnetically for 30-60min to completely dissolve the polymer;
[0074] Drug configuration in the inner layer of the drug stent: Weigh 30 mg of PTX and 10 mg of PLGA (molecular weight 20,000, PLA:PGA=80:20), add 10 ml of tetrahydrofuran respectiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com