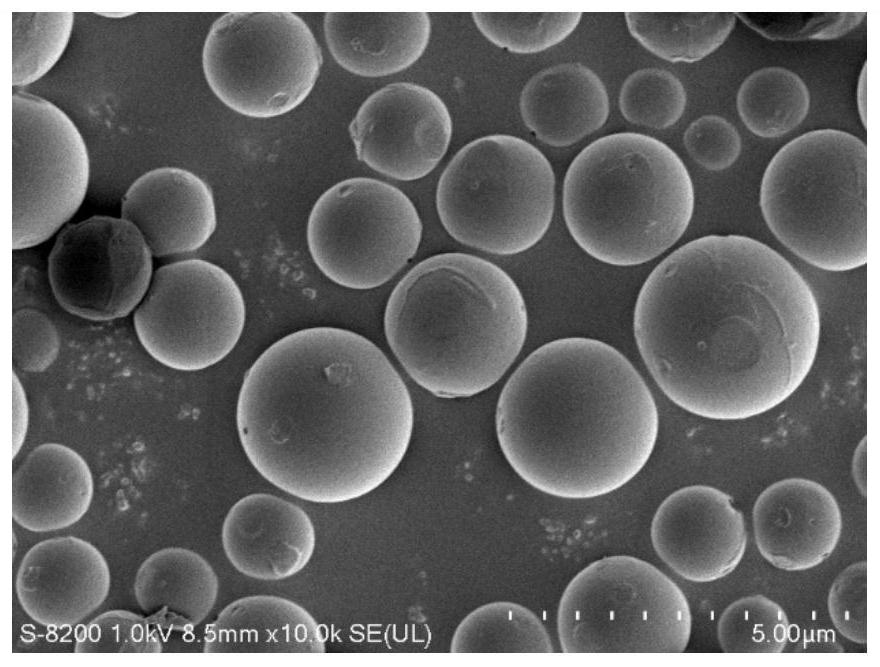
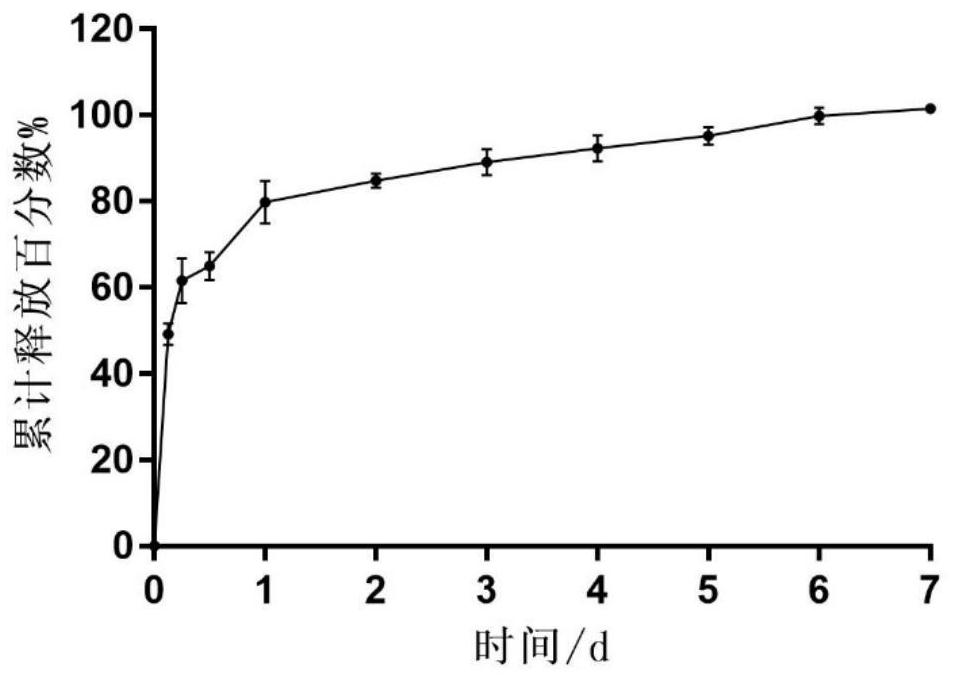
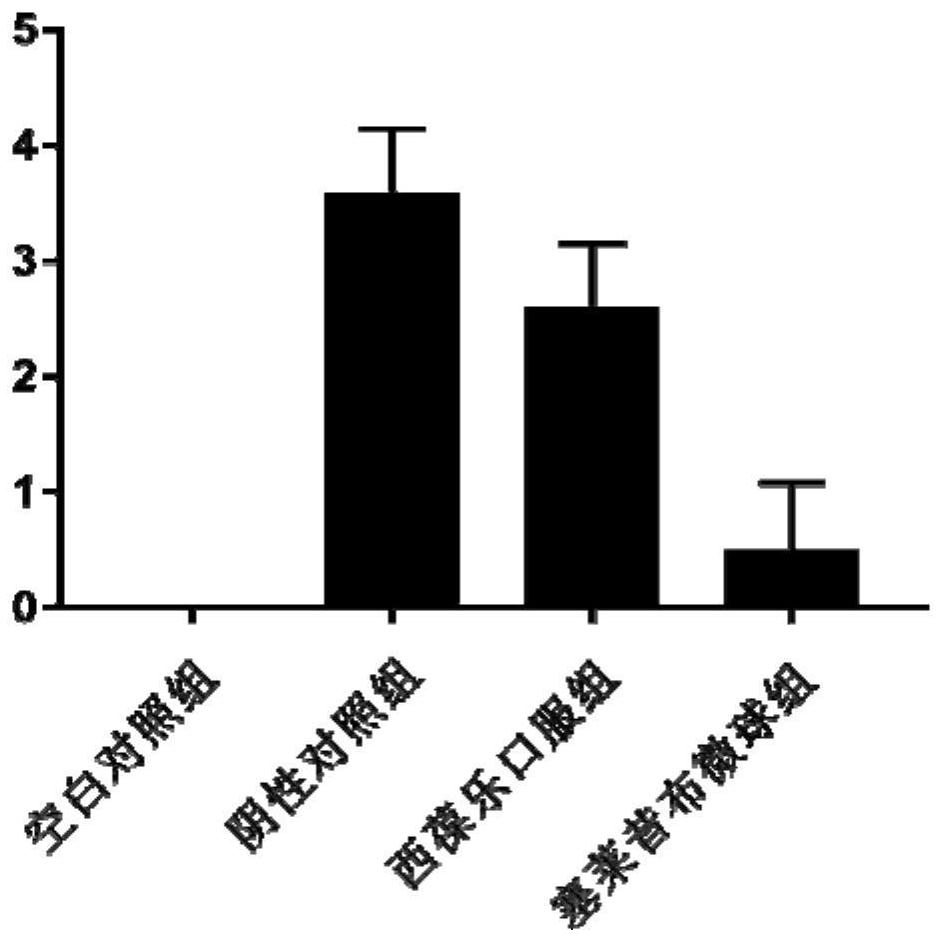
Celecoxib porous microspheres for articular cavity injection and preparation method of celecoxib porous microspheres
A technology of celecoxib and porous microspheres, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc. problem, to achieve the effect of simple preparation process, rapid deposition prevention, and maintenance of long-term swelling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of celecoxib PLGA microspheres
[0038] Weigh 0.18g PLGA7525, dissolve 60mg celecoxib (CEL) in 3mL dichloromethane as the organic phase, slowly drop into continuously stirred 30mL concentration of 2% PVA (w / v) aqueous solution, use a high-speed disperser at room temperature for 15 After emulsification at 7000rpm at ~25°C for 5min, pour 50mL of pure water into the above emulsion, stir magnetically at room temperature for 3h at 15-25°C, collect the microspheres by centrifugation, wash with pure water, and freeze-dry to obtain celecoxib PLGA Microspheres.
Embodiment 2~9
[0040] See Table 1 for the prescription.
[0041] The prescription of table 1 embodiment 2~9 celecoxib PLGA microsphere
[0042]
[0043] The microsphere preparation steps of Examples 2-9 are the same as Example 1.
Embodiment 10
[0045] Take by weighing 0.18g PLGA7525, 60mg CEL is dissolved in the mixed solvent (both volume ratio 20:1) 3mL of dichloromethane and ethyl acetate as the organic phase, slowly drip into the 30mL concentration of 2%PVA (w / v) Aqueous solution, use a high-speed disperser to emulsify at 7000rpm at room temperature 15-25°C for 5 minutes, pour 50mL of pure water into the above emulsion, stir magnetically at room temperature 15-25°C for 3 hours, centrifuge to collect the microspheres, wash with pure water, Freeze-dry to obtain celecoxib PLGA porous microspheres.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com