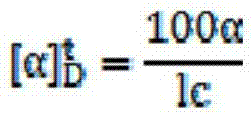Manufacture method for surgical implant-level poly(L-lactic acid)
A technology of surgical implantation and manufacturing method, applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems of uncontrolled insoluble particles, large monomer residue, small optical rotation, etc., to improve biocompatibility and reliability. Degradability, remarkable effect, and the effect of reducing particle pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
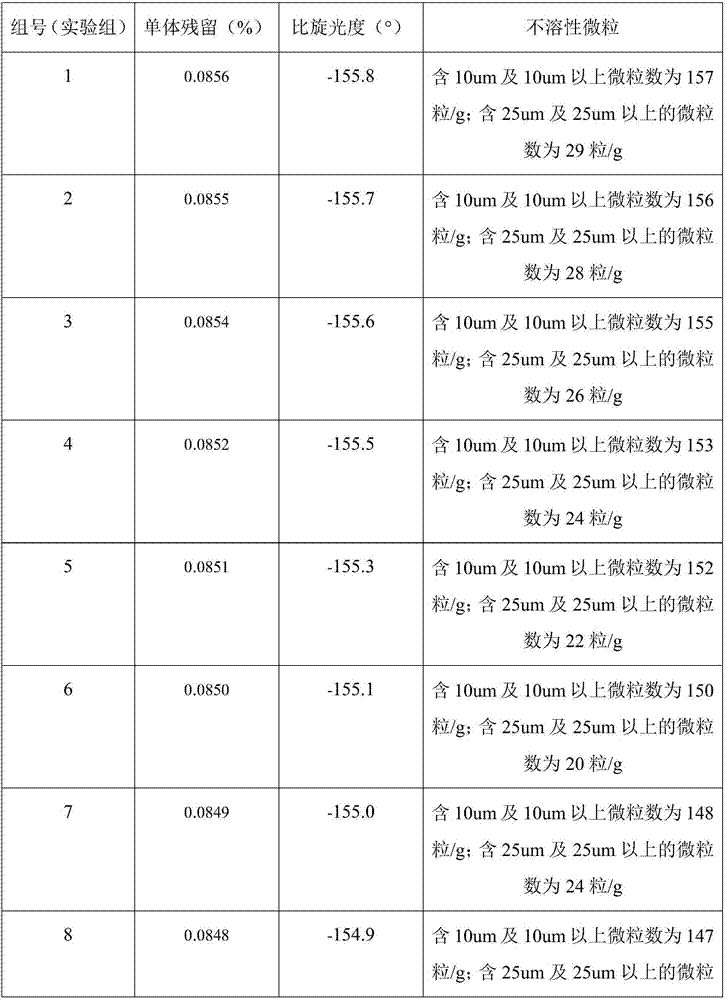
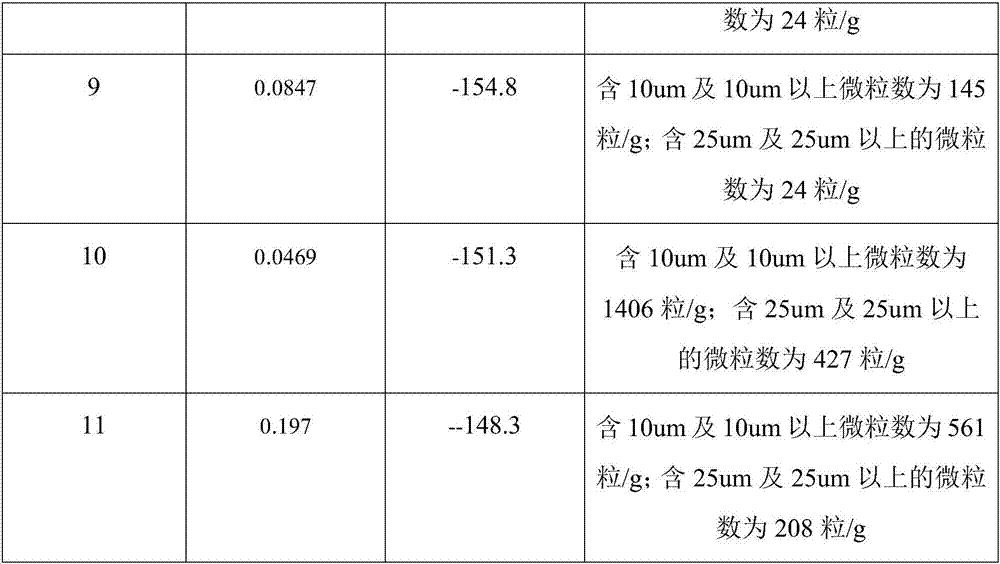
Examples
Embodiment 1
[0027] A kind of manufacture method of surgical implant grade poly-L-lactic acid, comprises the following steps:
[0028] Preparation of L-lactide: (1) Add 120kg of L-lactic acid into the reactor, keep it at 115°C under normal pressure for 1 hour, and remove the free water in the L-lactic acid; (2) Add Stir and mix 800g of stannous octoate evenly to disperse stannous octoate evenly in the system, raise the temperature to 150°C, and vacuumize to keep the vacuum degree ≤ -0.09MPa, dehydration and condensation for 4h; (3) After the dehydration polycondensation is completed, replace Keep the vacuum in the receiver, and quickly raise the temperature to 210°C, the product flows into the receiver and crystallizes rapidly, when the product is significantly reduced, stop the reaction; get the crude lactide; (4) After the crude lactide is cooled, add 120kg of ethyl acetate was heated to 40°C to dissolve it completely, placed in a low-temperature environment for recrystallization, and th...
Embodiment 2
[0031] A kind of manufacture method of surgical implant grade poly-L-lactic acid, comprises the following steps:
[0032] Preparation of L-lactide: (1) Add 180kg of L-lactic acid into the reactor, keep it at 105°C for 2 hours under normal pressure, and remove the free water in the L-lactic acid; (2) Add Stir and mix 1800g of stannous octoate evenly to disperse stannous octoate evenly in the system, raise the temperature to 140°C, and vacuumize to keep the vacuum degree ≤ -0.09MPa, dehydration and condensation for 6h; (3) After the dehydration polycondensation is completed, replace Keep the vacuum in the receiver, and quickly raise the temperature to 180°C, the product flows into the receiver and crystallizes quickly, when the product is significantly reduced, stop the reaction; get the crude lactide; (4) After the crude lactide is cooled, add 180kg of ethyl acetate was heated to 30°C to dissolve it completely, placed in a low temperature environment for recrystallization, and ...
Embodiment 3
[0035] A kind of manufacture method of surgical implant grade poly-L-lactic acid, comprises the following steps:
[0036]Preparation of L-lactide: (1) Add 1.2kg of L-lactic acid into the reactor, keep it at 120°C under normal pressure for 1.5h, remove the free water in the L-lactic acid; Add 6g of stannous octoate, stir and mix evenly, so that stannous octoate is uniformly dispersed in the system, heat up to 180 ° C, and vacuumize, keep the vacuum degree ≤ -0.09MPa, dehydration condensation 3h; (3) After dehydration polycondensation is completed , replace the receiver, keep the vacuum, and quickly heat up to 230 ° C, the product flows into the receiver and crystallizes quickly, when the product is significantly reduced, stop the reaction; the crude lactide is obtained; (4) after the crude lactide is cooled , add 2kg of ethyl acetate, heat to 20°C to dissolve it completely, place it in a low temperature environment for recrystallization, and then filter to obtain white flaky cr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com