Preparation method of fluorine-containing polysubstituted pyrrolidine derivative
A technology of fluorine-containing iodoalkane and pyrrolidine, which is applied in the field of preparation of fluorine-containing multi-substituted pyrrolidine derivatives, can solve the problems of many synthesis steps, high cost, and low yield, and achieve simple reaction operation and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
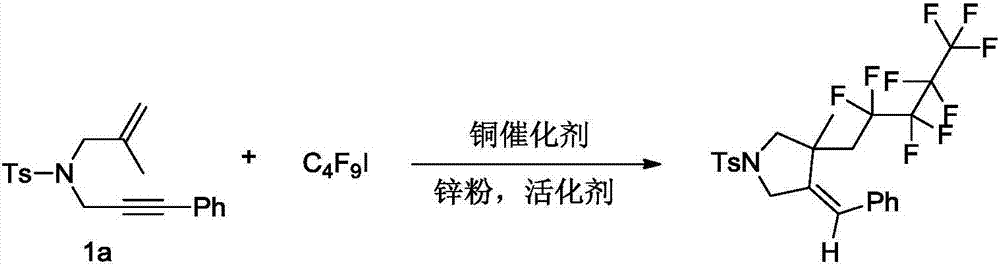
[0021] The preparation method of (E)-4-benzylidene-3-methyl-3-(2,2,3,3,4,4,5,5,5-nonafluoropentyl)-1-tosylpyrrolidine, its synthetic route is as follows:
[0022]
[0023] Embodiment one:
[0024] (E)-4-benzylidene-3-methyl-3-(2,2,3,3,4,4,5,5,5-nonafluoropentyl)-1-tosylpyrrolidine is prepared according to the following steps:
[0025] In an inert gas environment, add p-toluenesulfonamide enyne derivative 1a (1.0mmol, 339mg), perfluoroiodobutane (2.0mmol, 692mg), CuBr 2 (20%, 45mg), zinc powder (3.0mmol, 192mg), acetic acid (3.0mmol, 180mg), then add 10mL of dichloromethane, and react the reaction system in 40-50°C oil bath for 24 hours; The evaporator was rotated to remove the solvent, and the crude product was subjected to column chromatography to obtain 386 mg of fluorine-containing pyrrolidine compound, with a yield of 69%. The obtained compound has a melting point of 112-113.5°C, NMR 1 H NMR (400MHz, CDCl 3 ):7.74(d,J=8.0Hz,2H),7.38(d,J=8.0Hz,2H),7.25-7.27(m,3H),7.0...
Embodiment 2
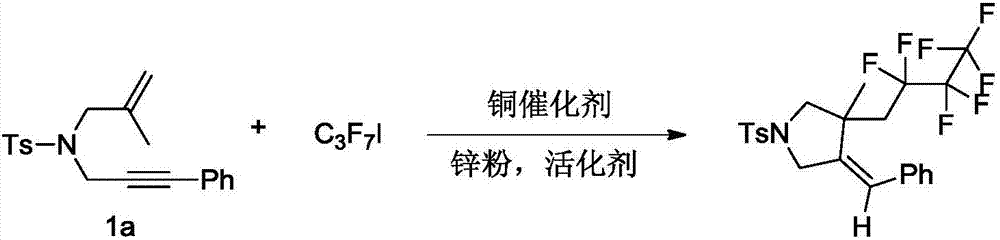
[0027] The preparation method of (E)-4-benzylidene-3-(2,2,3,3,4,4,4-heptafluorobutyl)-3-methyl-1-tosylpyrrolidine, its synthetic route is as follows:
[0028]
[0029] Embodiment two:
[0030] The preparation method of (E)-4-benzylidene-3-(2,2,3,3,4,4,4-heptafluorobutyl)-3-methyl-1-tosylpyrrolidine is carried out as follows:
[0031] In an inert gas environment, add p-toluenesulfonamide enyne derivative 1a (1.0mmol, 339mg), perfluoroiodopropane (2.0mmol, 592mg), CuBr 2 (20%, 45mg), zinc powder (3.0mmol, 192mg), acetic acid (3.0mmol, 180mg), then add 10mL of dichloromethane, and react the reaction system in 40-50°C oil bath for 24 hours; The evaporator was rotated to remove the solvent, and the crude product was subjected to column chromatography to obtain 356 mg of fluorine-containing pyrrolidine compound, with a yield of 70%. The obtained compound has a melting point of 94-95°C, NMR 1 H NMR (400MHz, CDCl 3 ):7.74(d,J=8.0Hz,2H),2.37(d,J=8.0Hz,2H),7.26-7.28(m,3H),7.08(d,...
Embodiment 3
[0033] (E)-3-methyl-3-(2,2,3,3,4,4,5,5,5-nonafluoropentyl)-4-(thiophen-2-ylmethylene)-1-tos ylpyrrolidine preparation method, Its synthetic route is as follows:
[0034]
[0035] Embodiment three:
[0036] (E)-3-methyl-3-(2,2,3,3,4,4,5,5,5-nonafluoropentyl)-4-(thiophen-2-ylmethylene)-1-tos ylpyrrolidine preparation method, Proceed as follows:
[0037] In an inert gas environment, add p-toluenesulfonamide enyne derivative 2a (1.0mmol, 345mg), perfluoroiodobutane (2.0mmol, 692mg), CuBr 2 (20%, 45mg), zinc powder (3.0mmol, 192mg), acetic acid (3.0mmol, 180mg), then add 10mL of dichloromethane, and react the reaction system in 40-50°C oil bath for 24 hours; The evaporator was rotated to remove the solvent, and the crude product was subjected to column chromatography to obtain 424 mg of fluorine-containing pyrrolidine compound with a yield of 75%. The resulting compound has a melting point of 100-102°C, and NMR 1 H NMR (400MHz, CDCl 3 ):7.73(d,J=8.0Hz,2H),7.38(d,J=8.0Hz,2H),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com