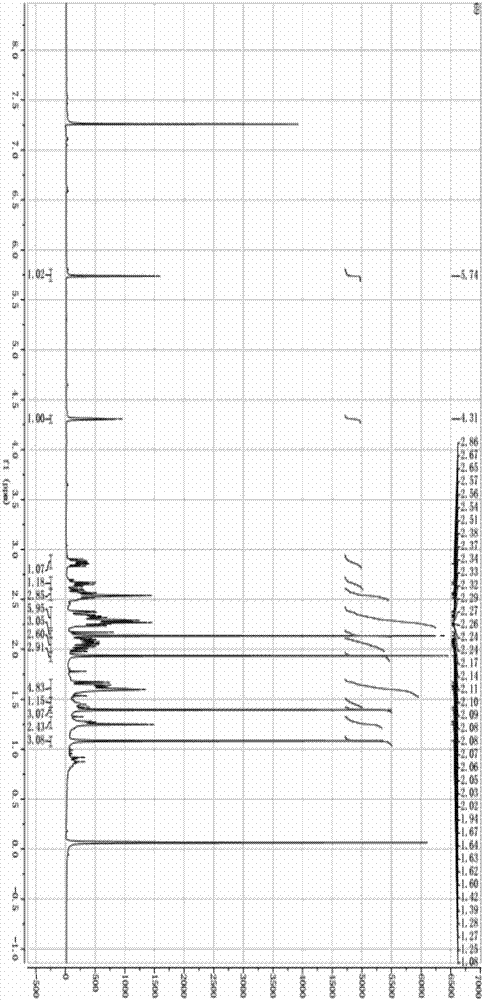
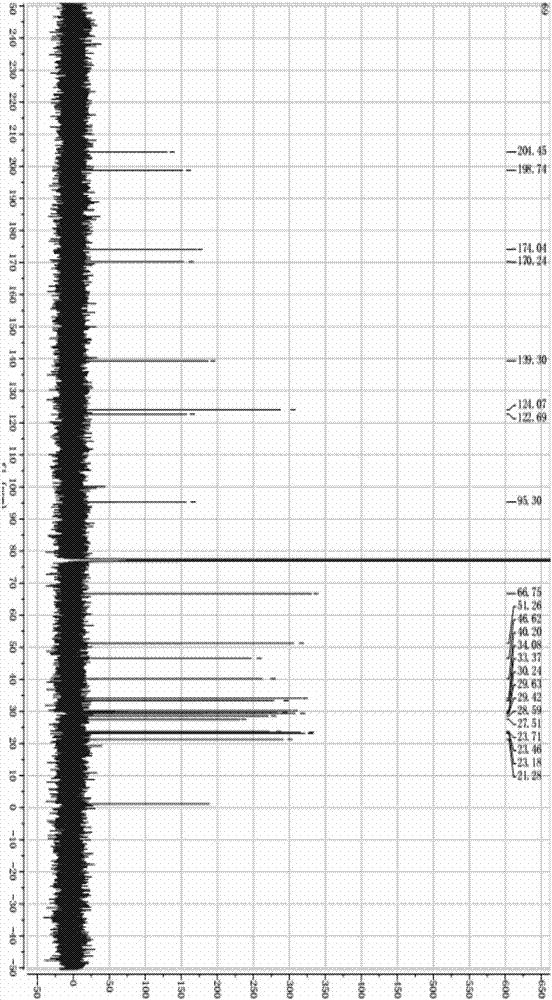
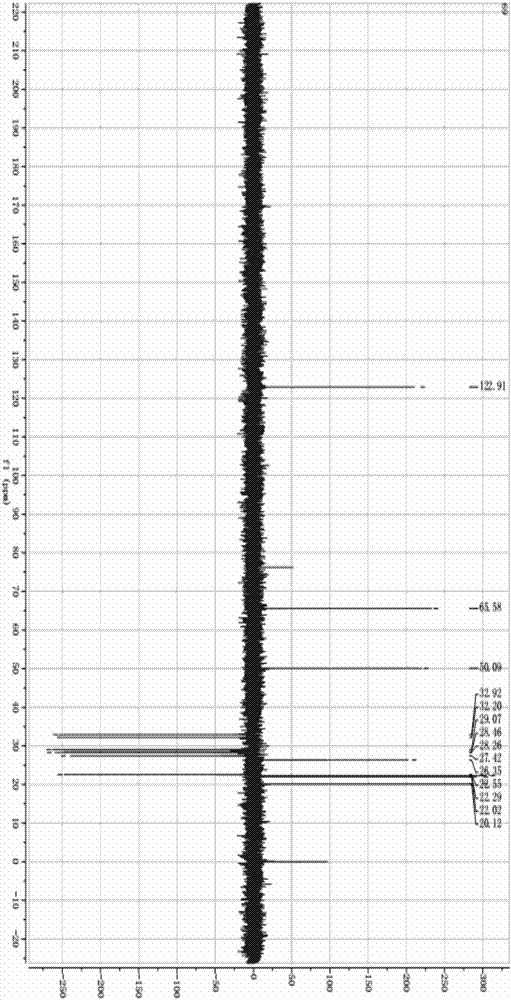
Preparation method of 17alpha-acetoxy-(8, 13)-ene-11alpha-hydroxyprogesterone
A kind of hydroxyprogesterone, acetoxy technology, applied in the field of compound preparation, can solve the problem of no synthesis method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] A preparation method of 17α-acetoxy-(8,13)-ene-11α-hydroxyprogesterone, operated according to the following steps:
[0085] (1) Preparation of Compound B
[0086] Add 200ml of THF and compound A (10g, 0.029mol) to the reaction flask, stir and cool down to about -40°C, slowly add phosphorus pentachloride (12g, 0.058mol), complete the addition within 30 minutes, and control the temperature at -30°C ~-35°C, react for 2 hours until the reaction is complete. Slowly add 5 times the amount of water dropwise, then add dropwise a solution of sodium hydroxide (10g, 0.25mol) / 18ml of water to adjust the pH to near neutral, concentrate THF under reduced pressure, and finally add 200ml of ice water. Filtered, washed with water, and dried at 60°C to obtain compound B (9.0g, 0.027mol), the molar yield was 94.9%, and the HPLC content was 98%, and the next step was carried out.
[0087] (2) Preparation of Compound C
[0088] Acetic anhydride (27.97 g, 0.27 mol) was added to the reacti...
Embodiment 2
[0097] A preparation method of 17α-acetoxy-(8,13)-ene-11α-hydroxyprogesterone, operated according to the following steps:
[0098] (1) Preparation of Compound B
[0099]Add 200ml of diethyl ether and compound A (10g, 0.029mol) to the reaction flask, stir and cool down to about -40°C, slowly add phosphorus trichloride (7.96g, 0.058mol), complete the addition within 30 minutes, and control the temperature at -30°C ℃~-35℃, react for 2 hours until the reaction is complete. A solution of sodium hydroxide (10 g, 0.25 mol) / 18 ml of water was added dropwise to adjust the pH to near neutral, and finally 200 ml of ice water was precipitated. Filtered, washed with water, and dried at 60°C to obtain compound B (9.1g, 0.028mol), the molar yield was 95.6%, and the HPLC content was 97.8%, and the next step was carried out.
[0100] (2) Preparation of Compound C
[0101] Add acetic anhydride (27.97g, 0.27mol) to the reaction flask, add compound B (9.0g, 0.027mol), stir and raise the temper...
Embodiment 3
[0110] A preparation method of 17α-acetoxy-(8,13)-ene-11α-hydroxyprogesterone, operated according to the following steps:
[0111] (1) Preparation of Compound B
[0112] Add 200ml of tetrahydrofuran and compound A (10g, 0.029mol) to the reaction flask, stir and cool down to about -40°C, slowly add phosphorus oxychloride (10g, 0.065mol), complete the addition within 30 minutes, and control the temperature at -20°C ~-40°C, react for 1-2 hours until the reaction is complete. Slowly add 5 times the amount of water dropwise, control the temperature ≤ 20°C, then add dropwise a solution of sodium hydroxide (10g, 0.25mol) / 18ml of water, adjust the pH to near neutral, concentrate THF under reduced pressure, and finally precipitate into 200ml ice water. Filter, wash with water, and dry at 60° C. to obtain compound B (9.0 g, 0.027 mol), the molar yield is 94.9%, and the HPLC content is 97%, and proceeds to the next step.
[0113] (2) Preparation of Compound C
[0114] Add acetic anhy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com