Synthetic method of omega-substituted biuret compound
A synthesis method and biuret technology are applied in the synthesis field of ω-substituted biuret compounds, can solve the problems of inability to transport, cumbersome steps, long time and the like, and achieve reduced risk, simple operation and good applicability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the synthesis of N-phenylbiuret
[0044] Add 102mg of aniline (1.1mmol), 428mg of potassium cyanate (5.28mmol), 6ml of water, 6ml of acetonitrile into a 25ml reaction vessel, stir and heat up to 80°C, add 2.64mmol of acetic acid, add 2.64mmol of acetic acid after 1 hour of reaction, and stir at constant temperature Reaction 10h. The reaction solution was cooled to room temperature, extracted with ethyl acetate (3×40ml), the ethyl acetate phases were combined, washed with 40ml of water and 40ml of saturated brine, dried over anhydrous sodium sulfate, and the ethyl acetate was recycled. Column chromatography (silica gel, 200-300 mesh; developer, ethyl acetate: dichloromethane: ethanol = 40:40:1) obtained 0.15 g of N-phenylbiuret with a yield of 76%.
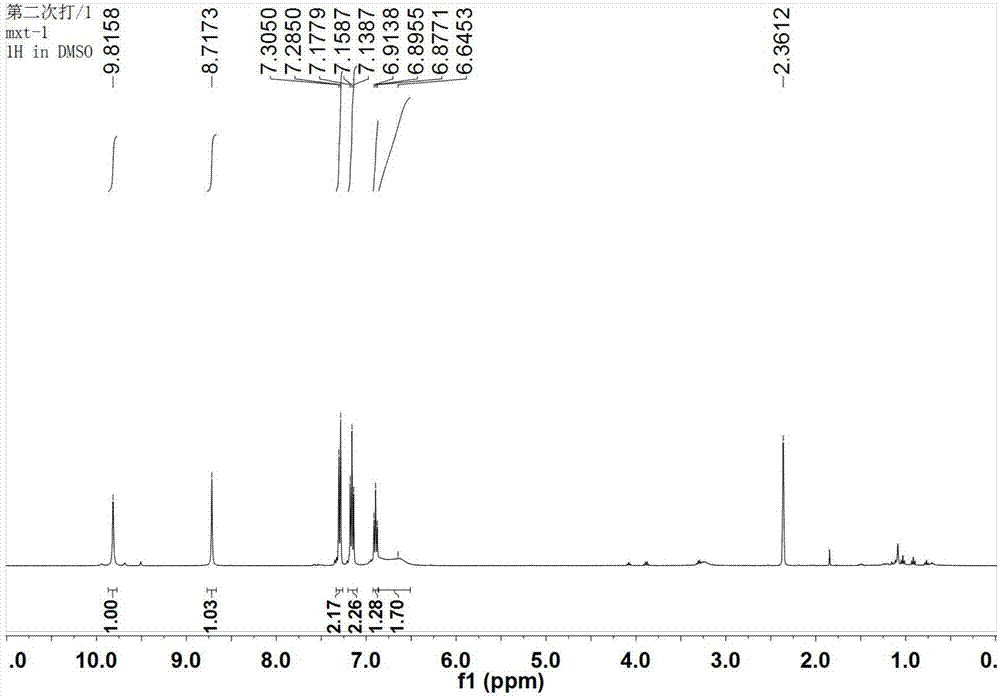
[0045] N-phenylbiuret, white powder, melting point: 184-185℃. 1 H NMR (400MHz, DMSO-d 6 )δ: 9.82(s, 1H), 8.72(s, 1H), 7.29(d, J=8.0Hz, 2H), 7.16(t, J=7.8Hz, 2H), 6.90(t, J=7.3Hz, 1H),6.65(s,2H); 13 C NMR (...
Embodiment 2
[0046] Embodiment 2: the synthesis of N-(4-iodobenzene)-biuret
[0047]Add 241mg p-iodoaniline (1.1mmol), 428mg potassium cyanate (5.28mmol), 6ml water, 6ml N'N-dimethylformamide into a 25ml reaction vessel, stir and heat up to 80°C, add 2.64mmol acetic acid for 1 hour Then add 2.64mmol acetic acid and stir at constant temperature for 10h. The reaction solution was cooled to room temperature, extracted with ethyl acetate (3×40ml), the ethyl acetate phases were combined, washed with water (40ml) and saturated brine (40ml) successively, dried over anhydrous sodium sulfate, and the ethyl acetate was recycled. Column chromatography (silica gel, 200-300 mesh; developing solvent, ethyl acetate:dichloromethane:ethanol=40:40:1) obtained N-(4-iodobenzene)-biuret 0.277g, yield 83% .
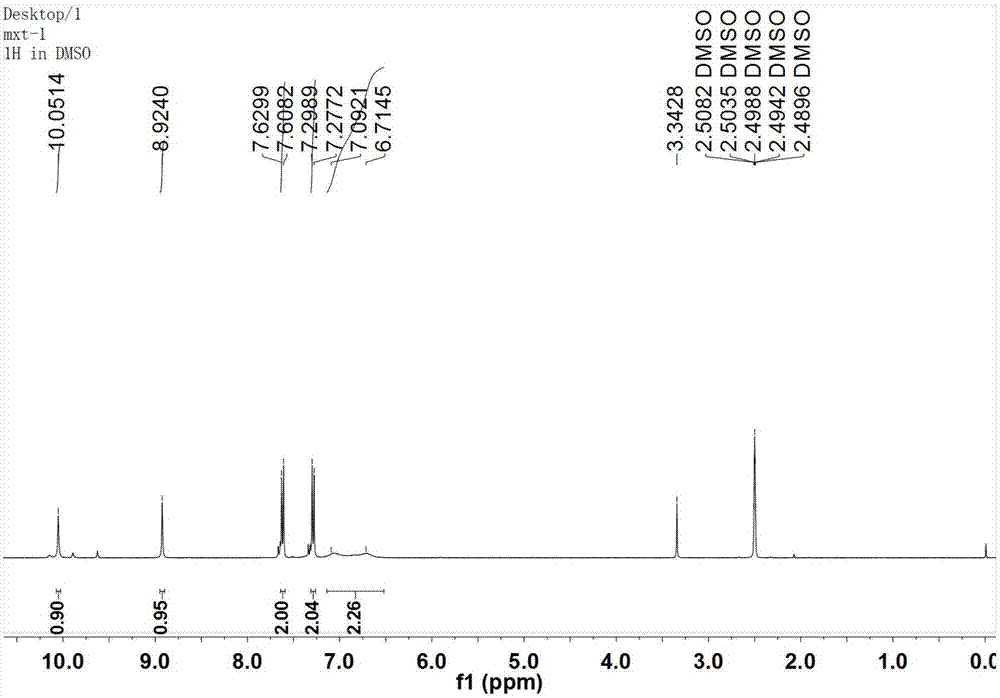
[0048] N-(4-iodobenzene)-biuret, white powder, melting point: 296-297℃. 1 H NMR (400MHz, DMSO-d 6 )δ:10.05(s,1H),8.92(s,1H),7.62(d,J=8.7Hz,2H),7.29(d,J=8.7Hz,2H),6.90(d,J=151.1Hz, 2H); 13 C NMR (101M...
Embodiment 3
[0049] Embodiment 3: the synthesis of N-(4-bromobenzene)-biuret
[0050] Add 189mg p-bromoaniline (1.1mmol), 642mg potassium cyanate (7.92mmol), 6ml water, 4ml ethanol, stir and heat up to 70°C, add acetic acid (3.96mmol), and add acetic acid (3.96mmol) after 1 hour in a 25ml reaction vessel. ), stirring at constant temperature for 10h. The reaction solution was cooled to room temperature, extracted with ethyl acetate (3×40ml), the ethyl acetate phases were combined, washed with water (40ml) and saturated brine (40ml) successively, dried over anhydrous sodium sulfate, and the ethyl acetate was recycled. Column chromatography (silica gel, 200-300 mesh; developing solvent, ethyl acetate:dichloromethane:ethanol=40:40:1) obtained N-(4-bromobenzene)-biuret 0.234g, yield 83% .
[0051] N-(4-bromobenzene)-biuret, white powder, melting point: 293-294℃. 1 H NMR (400MHz, DMSO-d 6 )δ: 10.09(s, 1H), 8.94(s, 1H), 7.4'8(d, J=8.9Hz, 2H), 7.43(d, J=9.0Hz, 2H), 6.91(d, J=145.4 Hz,2H); ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com