A purification method and application of porcine circovirus type 2 virus-like particles
A technology of porcine circovirus and type 2 virus, applied in the direction of microorganism-based methods, biochemical equipment and methods, antiviral agents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 is the preparation process of the porcine circovirus type 2 semi-finished product in the purification method according to the present invention.
[0021] The nucleotide sequence encoding the porcine circovirus type 2 virus-like particle gene is connected to the baculovirus to construct a recombinant baculovirus seed, the virus seed infects insect cells (Sf9 cells), and the Sf9 cell culture conditions are 27 ° C, 140 rpm , 96h~120h. When the cells were lysed to release the virus, the venom was collected and inactivated by binary ethyleneimine (BEI) to obtain porcine circovirus type 2 virus-like particles.
[0022] The inactivated venom was clarified to remove the precipitate with 0.2M Na 2 HPO 4 Adjust the pH of the semi-finished product to about 6.5-6.8 to obtain the semi-finished product before purification of porcine circovirus type 2 virus-like particles.
Embodiment 2
[0023] Example 2 Purification of porcine circovirus type 2 virus-like particles
[0024] 1) Strong cation exchange chromatography
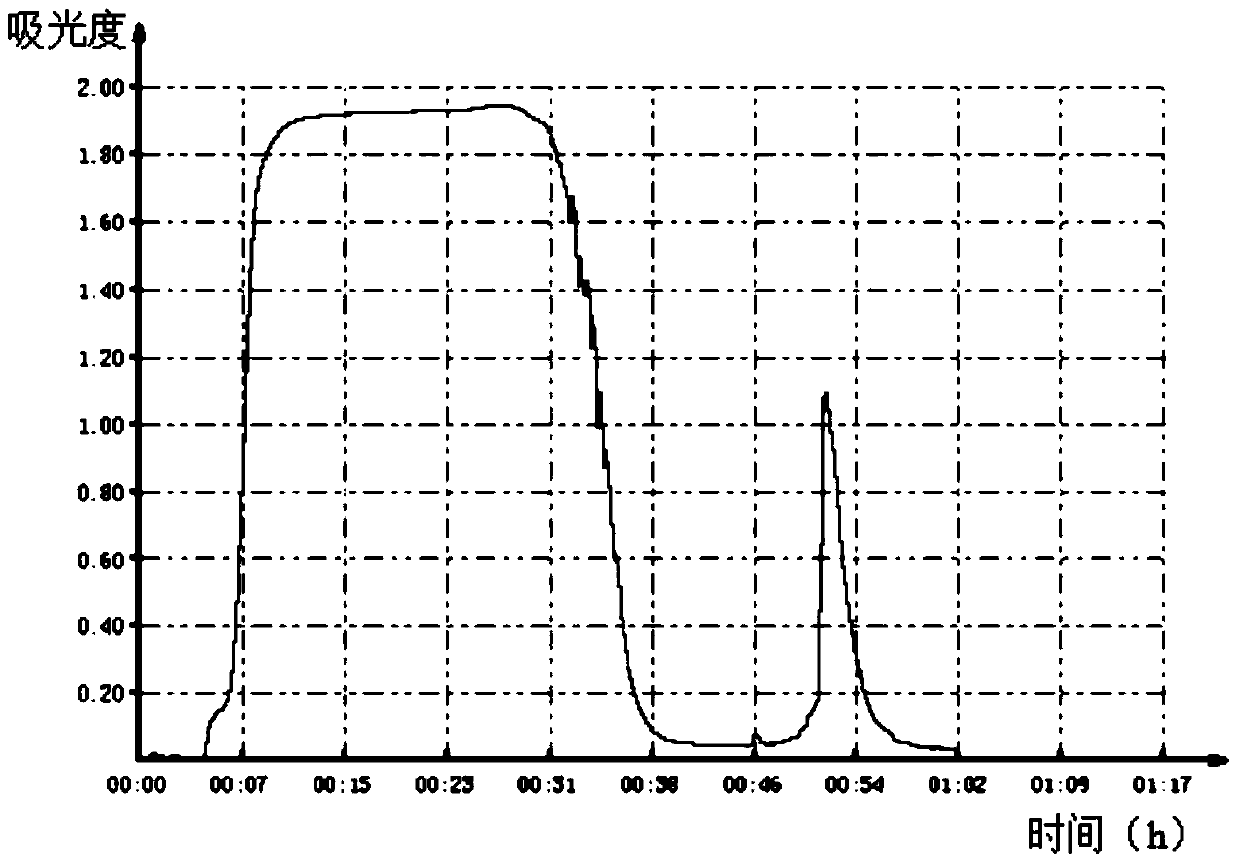
[0025] Add the pretreated semi-finished product to a strong cation exchange column equilibrated with PBS buffer A (20mM PBS, 0.1M NaCl, pH=6.8), and use PBS buffer B (20mM PBS, 1.0M NaCl, pH=8.0 ) for elution, the elution volume is 3 column volumes, the flow rate is 2.0ml / min, and each peak component is collected, the strong ion exchange chromatogram is as follows figure 1 shown.
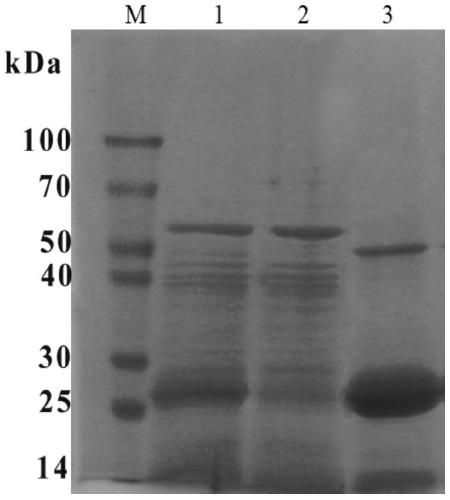
[0026] 2) Polyacrylamide gel electrophoresis (SDS-PAGE) to determine the active protein components
[0027] Take 80 μl of samples from each stage in a 1.5ml EP tube, then add 20 μl of 5×SDS-PAGE loading buffer, and mix well. Each EP tube was placed in a boiling water bath for 5 minutes, and centrifuged at 4000 rpm for 3 minutes to obtain SDS-PAGE samples. The SDS-PAGE of each component was as follows: figure 2 as shown, figure 2 Middle, lane M: protein marker; la...
Embodiment 3
[0033] Example 3 is a safety test of a vaccine containing porcine circovirus type 2 virus-like particles obtained by the above-mentioned purification method of porcine circovirus type 2 virus-like particles.
[0034] 1. Experimental Vaccine Vaccines were prepared by adding the same appropriate amount of adjuvant to samples before and after purification of porcine circovirus type 2 virus-like particles.
[0035] 2. Test piglets were purchased from Yunli Animal Husbandry Co., Ltd., Dantu District, Zhenjiang City, and all of them were negative for porcine circovirus type 2 antigen antibodies.
[0036] 3. Experimental method
[0037] 3.1 Single-dose vaccination safety experiment
[0038] Piglets aged 2 to 3 weeks were selected, and 200 piglets were injected intramuscularly with vaccines prepared from viruses before and after purification, 1ml / head. After the injection, observe the mental state, drinking water, and food intake of pigs in each group every day; whether there is an ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com