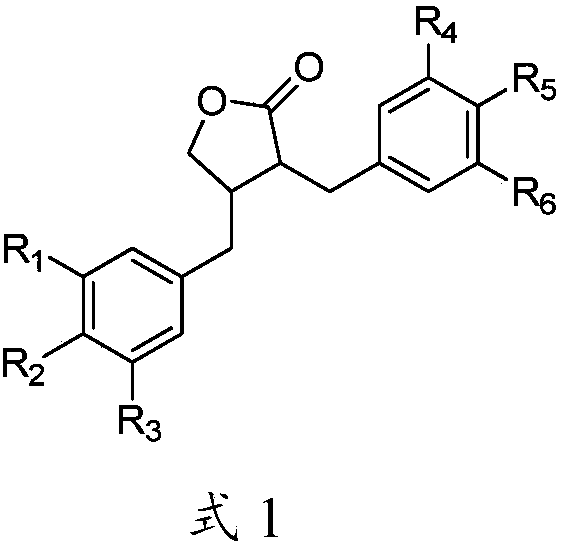
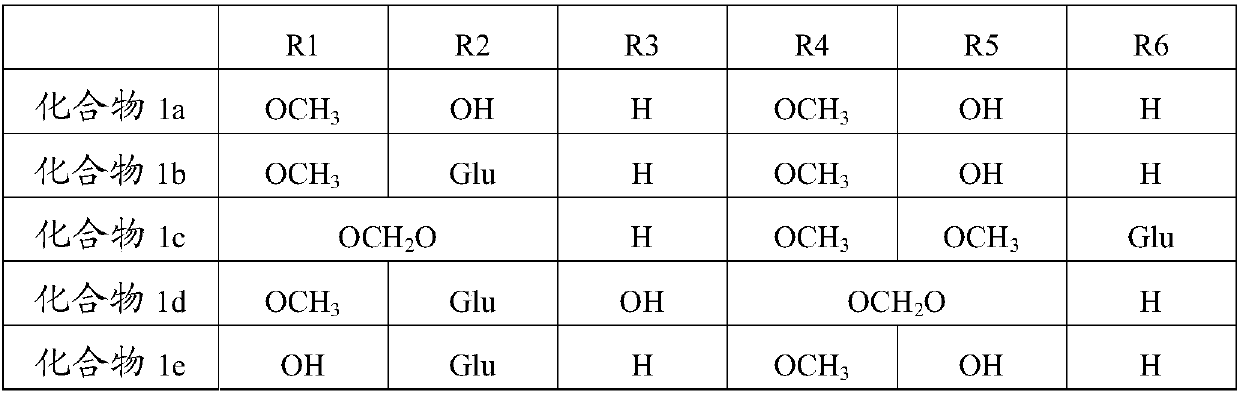
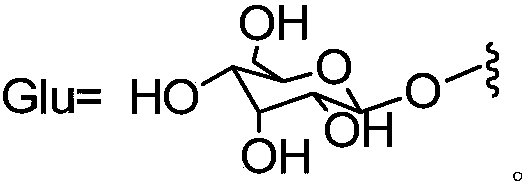
Application of lignans compound in preparation of anti-hepatic fibrosis medicine
A technology of lignans and compounds, which is applied in the application field of lignans in the preparation of anti-hepatic fibrosis drugs, can solve the problems that lignans do not form a system, lack batch or large-scale preparation methods, etc., and achieve Strong anti-hepatic stellate cell proliferation, good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of embodiment 1 compound
[0035] The lignan compounds described in this example are extracted from natural Bupleurum chinensis, and the specific process is as follows:
[0036] 1) Take 15 kg of dried bamboo-leaf Bupleurum chinensis (produced in Yunnan, China) and grind it into a coarse powder, heat and reflux extraction with 9 times the amount of 75% ethanol for 3 times, each time for 1 hour, filter, combine the filtrates, concentrate under reduced pressure to remove ethanol, Stir and add water to dilute to 40L, and sequentially extract with petroleum ether, ethyl acetate, n-butanol and water. The extracts were collected and rotovaped to obtain individual fractions.
[0037] 2) Dissolve the extracted part of ethyl acetate in ethyl acetate to make the concentration reach 20-50 mg / ml, pass through 200-300 mesh silica gel column chromatography, use petroleum ether-ethyl acetate system with 4:1 (v / v) , 1:1 (v / v), 1:8 (v / v), 1:20 (v / v), pure methano...
Embodiment 2
[0052] Example 2 In vitro inhibition of activated hepatic stellate cells by MTT method
[0053] In this example, the hepatic stellate cell experiment of in vitro inhibition by MTT method was carried out for the lignan compounds described in the present invention.
[0054] Experimental materials: human hepatic stellate cell line LX-2, rat hepatic stellate cell line T-6
[0055] Drugs to be tested: 15 compounds were prepared with final concentration gradients of 100 μM, 20 μM, 4 μM, 0.8 μM and 0.16 μM.
[0056] Positive control: Saikosaponin d was prepared with final concentration gradients of 100 μM, 20 μM, 4 μM, 0.8 μM and 0.16 μM.
[0057] Taking human hepatic stellate cell line LX-2 as an example, the inhibitory effect of 15 compounds on activated hepatic stellate cells was determined by MTT method.
[0058] (1) Count the LX-2 human hepatic stellate cell line, and make the cells 5×10 4 cells / mL of cell suspension.
[0059] (2) Take the cell suspension and inoculate it in...
Embodiment 3
[0069] Example 3 Anti-hepatic fibrosis experiment in rats
[0070] In this example, an in vivo anti-hepatic fibrosis experiment in rats was carried out for the compound described in the present invention.
[0071] Experimental animals: male SD rats, SPF grade, 6-8 weeks old, weighing 180+-20, purchased from the Experimental Animal Center of the Chinese Academy of Military Medical Sciences, certificate number: SCXK-(Army) 2012-2004. Normal adaptive feeding Conditions: Breeding in independent isolation cages, temperature 18-21°C, humidity >40%, 150Pa pressurized air supply, cycle light (12h light, 12h dark), free access to water and food (standard pellet feed), change the pad every other day feed and fodder.
[0072] Experimental grouping: 265 experimental rats were randomly divided into 18 groups, which were normal group (10 rats), model group (15 rats), compound 1a group (15 rats), compound 1b group (15 rats), compound 1c group ( 15 rats), compound 1d group (15 rats), compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com