Azopyridines salt compound and preparation method
A technology of azopyridinium salt compound and azopyridine, which is applied in the field of azopyridinium salt compound and its preparation, and achieves the effect that the preparation method is simple and easy to be popularized and applied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
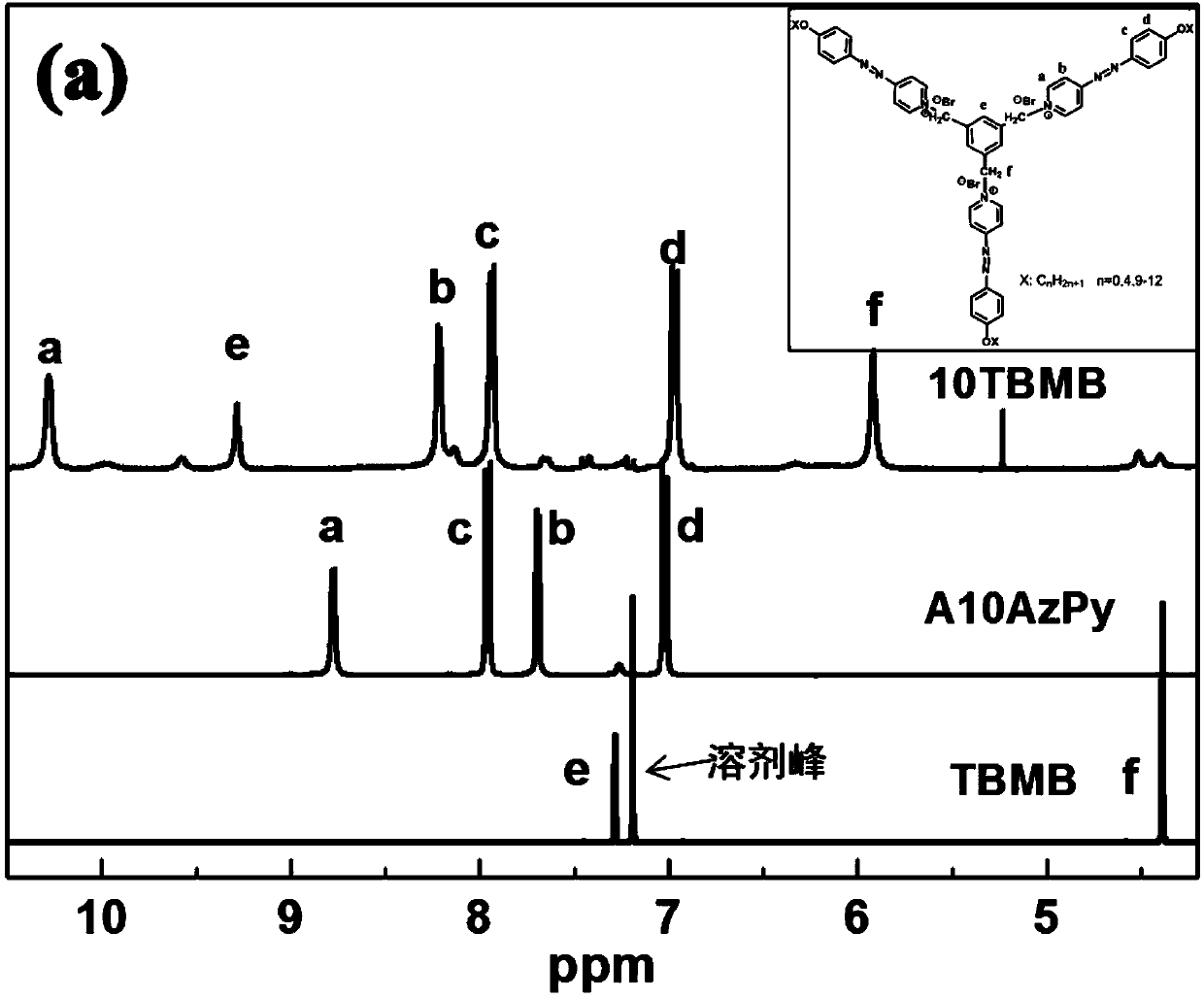
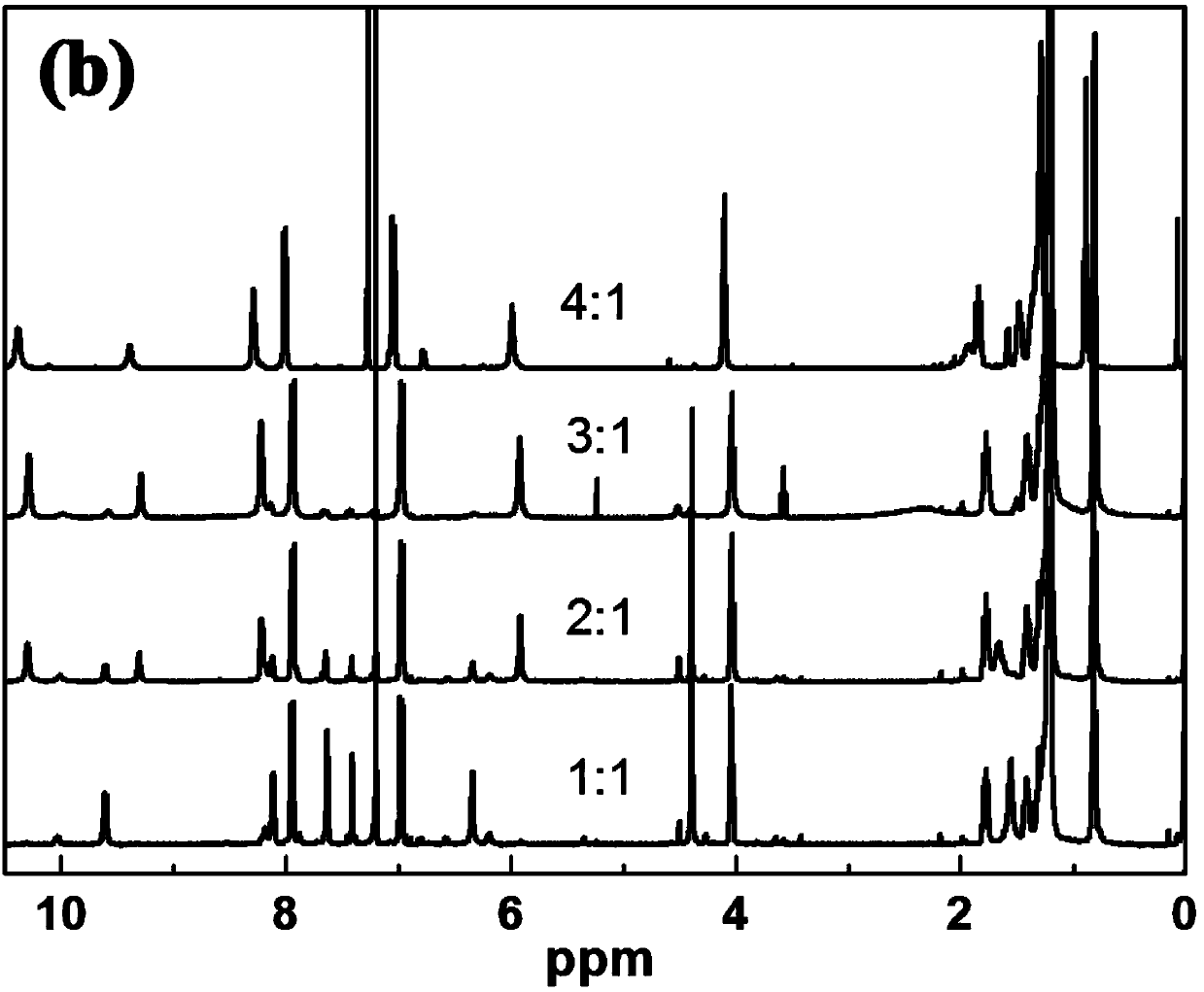
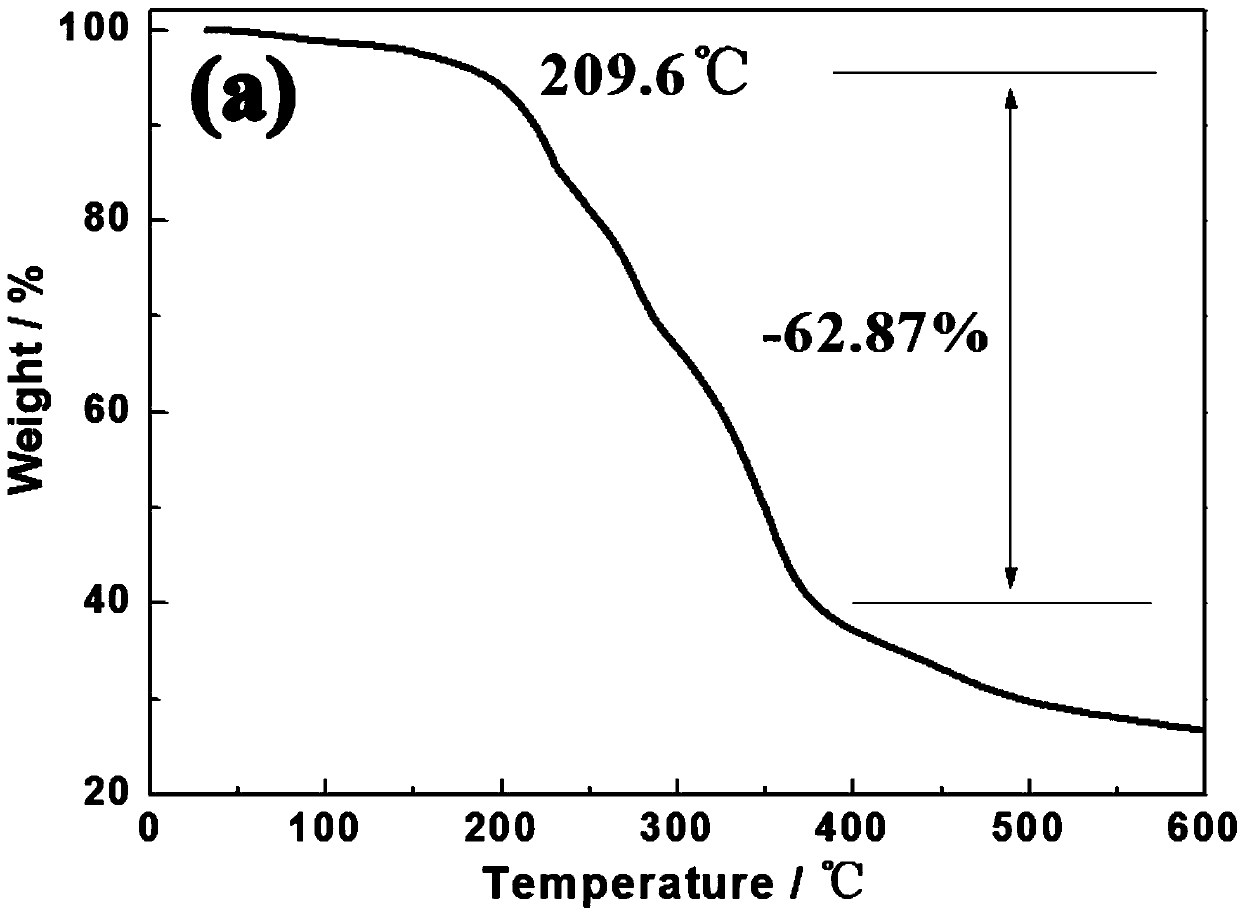
[0037] Embodiment 1: the synthesis of compound 1d
[0038]
[0039] 1. Synthesis of intermediate 4-hydroxyazopyridine
[0040] 4.00 g (58 mmol) of sodium nitrite and 5.00 g (53 mmol) of phenol were placed in 10% (w / w) aqueous sodium hydroxide solution (20 mL), and cooled to 0°C. The above mixed solution was added dropwise to 45 mL of hydrochloric acid solution (25 mL of 11N hydrochloric acid and 20 mL of water) containing 6.00 g (64 mmol) of 4-aminopyridine. Stir under ice bath for 0.5h. Then, the pH value of the reaction mixture was adjusted to 6-7 with 10% (w / w) aqueous sodium hydroxide solution. Filtration yielded a yellow precipitate. Washed with water, recrystallized and dried to obtain a yellow solid. Yield: 38.0%.
[0041] 2. Synthesis of azopyridine derivatives (referred to as A10AzPy)
[0042] 4-Hydroxyphenylazopyridine (2.0 g, 0.1 mol), potassium iodide (0.005 g) and potassium carbonate (6.9 g, 0.005 mol) were dissolved in DMSO (20 ml) solution. A solution ...
Embodiment 2
[0045] Embodiment 2: the synthesis of compound 1a
[0046]
[0047] The reaction steps are the same as in Example 1, except that in step 2, bromononane is used instead of chlorodecane to obtain azopyridine derivatives (referred to as A9AzPy)
Embodiment 3
[0048] Embodiment 3: the generation of compound 1b
[0049]
[0050]
[0051] The reaction steps are the same as in Example 1, except that n-chlorodecane is replaced by n-bromobutane in step 2 to obtain azopyridine derivatives (referred to as A4AzPy).
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com