A ratiometric bifunctional fluorescent probe based on coumarin dye and its synthesis and application
A technology of coumarin dye and fluorescent probe, which is applied in the field of base coumarin dye ratio bifunctional fluorescent probe and its synthesis and application, can solve the problem of limited probe application range, low fluorescence quantum yield, and reaction time Long and other problems, to achieve the effects of efficient identification and measurement, efficient quantitative identification, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] (1) Synthesis of intermediate compound 1
[0057] Add 10.0g (56.8mmol) 7-hydroxy-4-methylcoumarin and 18.4g (130nmol) urotropine to a 250mL three-necked round-bottomed flask, then add 80mL of glacial acetic acid, dissolve it by ultrasonic, and keep the temperature of the reaction solution Reflux and stir at 90°C for 6 hours, then add 20 mL of hydrochloric acid dropwise with a dropper, continue to reflux and stir at the same temperature for 50 minutes, then cool the reaction solution to room temperature, slowly add 100 mL of ice water, and extract three times with ether (3×100 mL) , dried the organic phase with anhydrous sodium sulfate, and rotary evaporated under reduced pressure to obtain a crude product, which was purified by a column with an eluent (petroleum ether / dichloromethane=10 / 1) to obtain a light yellow solid.
[0058] (2) Synthesis of probes
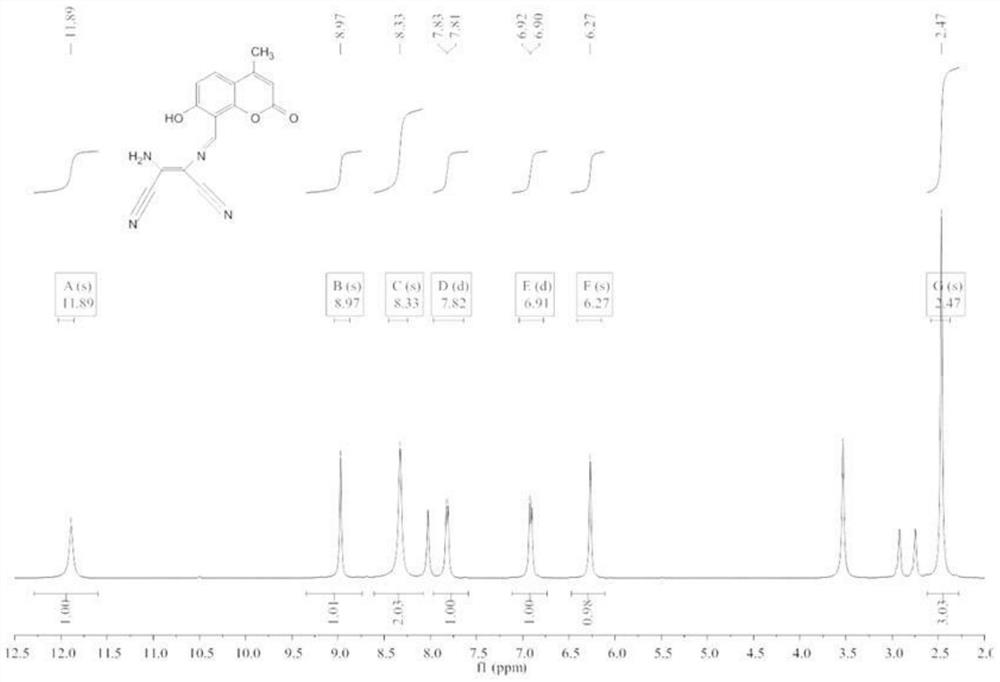
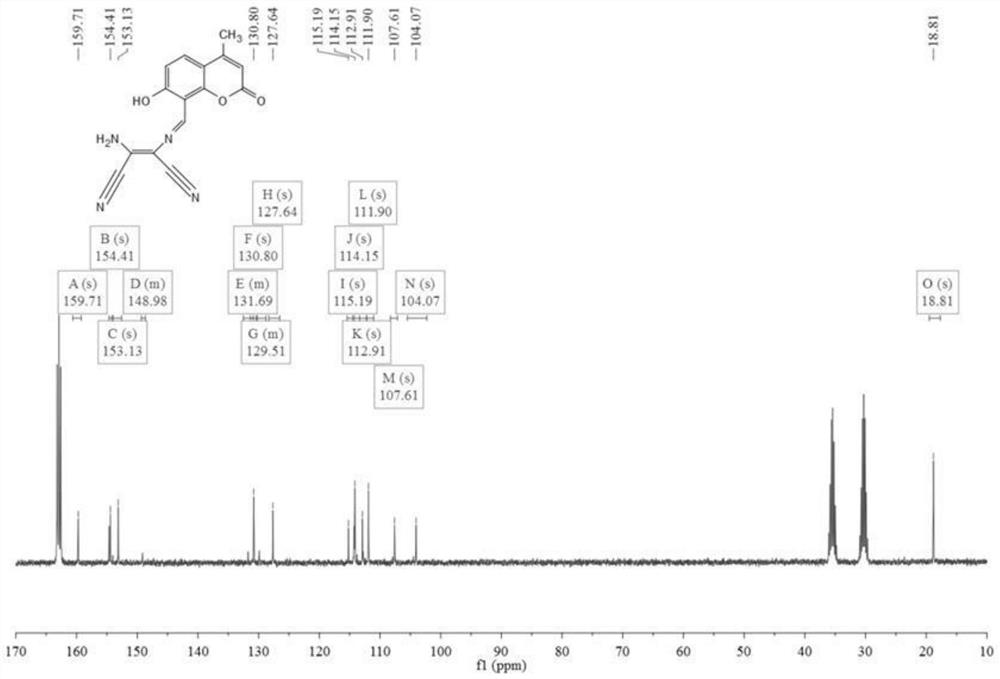
[0059] Weigh 326mg (1.6mmol) of compound 1 and 216mg (2mmol) of diaminomaleonitrile, dissolve them in 30mL of ethan...
Embodiment 2
[0061] (1) Synthesis of intermediate compound 1
[0062] Add 8g of 7-hydroxy-4-methylcoumarin and 10g of urotropine to a 250mL three-necked round-bottomed flask, then add 60mL of glacial acetic acid, dissolve it with ultrasonic waves, keep the temperature of the reaction solution at 90°C and reflux for 6 hours, then use Add 20mL of hydrochloric acid dropwise through the dropper, continue to reflux and stir at the same temperature for 50 minutes, then cool the reaction solution to room temperature, slowly add 100mL of ice water, extract three times with ether (3×100mL), and dry the organic phase with anhydrous sodium sulfate , and rotary evaporation under reduced pressure to obtain a crude product, which was purified through a column with an eluent (petroleum ether / dichloromethane=10 / 1) to obtain a light yellow solid.
[0063] (2) Synthesis of probes
[0064] Weigh 300mg of compound 1 and 200mg of diaminomaleonitrile, dissolve them in 25mL of ethanol / water solution, add 4 drop...
Embodiment 3
[0066] (1) Synthesis of intermediate compound 1
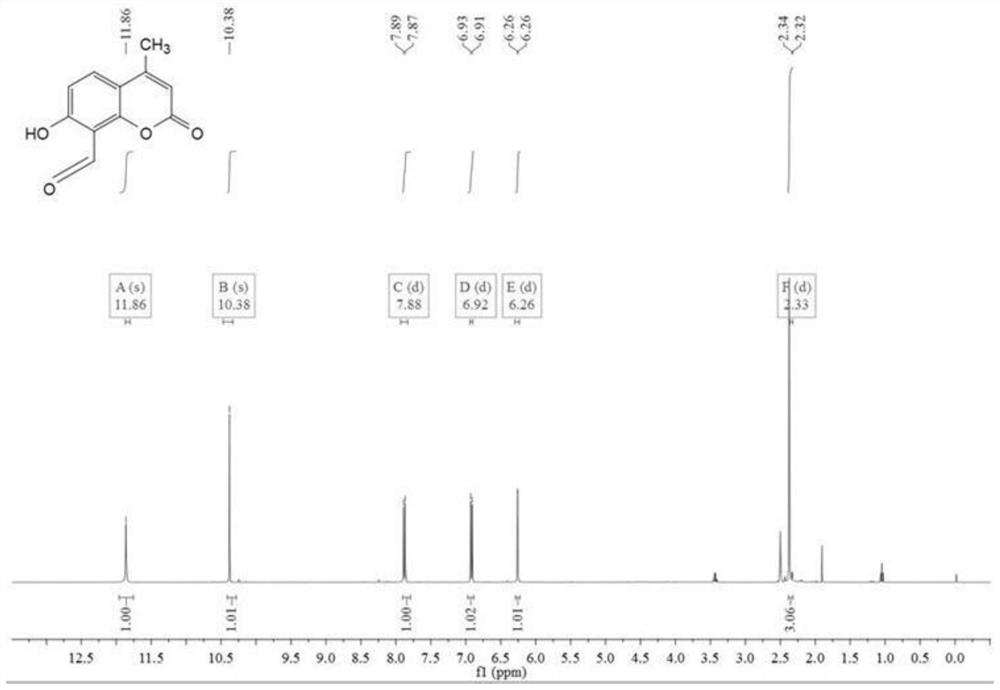
[0067] Add 5.0g (28.4mmol) 7-hydroxy-4-methylcoumarin and 9.2g (65nmol) urotropine to a 250mL three-necked round-bottomed flask, then add 40mL of glacial acetic acid, dissolve it by ultrasonic, and keep the temperature of the reaction solution Reflux and stir at 90°C for 6 hours, then add 20 mL of hydrochloric acid dropwise with a dropper, continue to reflux and stir at the same temperature for 50 minutes, then cool the reaction solution to room temperature, slowly add 100 mL of ice water, and extract three times with ether (3×100 mL) , the organic phase was dried with anhydrous sodium sulfate, and the crude product was obtained by rotary evaporation under reduced pressure, and the eluent (petroleum ether / dichloromethane=10 / 1) was purified by column to obtain a light yellow solid, that is, compound 1 was 0.84 mg, the yield 14.5%. H NMR spectrum see figure 1 .
[0068] Compound 1 1 H NMR picture as figure 1 Shown: 1 H NMR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com