Organometallic complex as well as preparation method and application thereof
A technology of organic metals and complexes, applied in indium organic compounds, platinum group organic compounds, organic chemistry, etc., can solve the unsolved problems of blue phosphorescent materials, achieve good luminescence performance, wide spectrum application range, and low mass production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0111] The embodiment of the present invention also proposes the preparation method of the organometallic complex, which at least includes the following steps:
[0112] The precursor material L and iridium chloride (IrCl 3 ) The dimer is prepared by reaction, and the dimer is reacted with the ligand compound to obtain the compound represented by the general formula I;
[0113] The chemical reaction equation is as follows:
[0114]
[0115]
Synthetic example 1
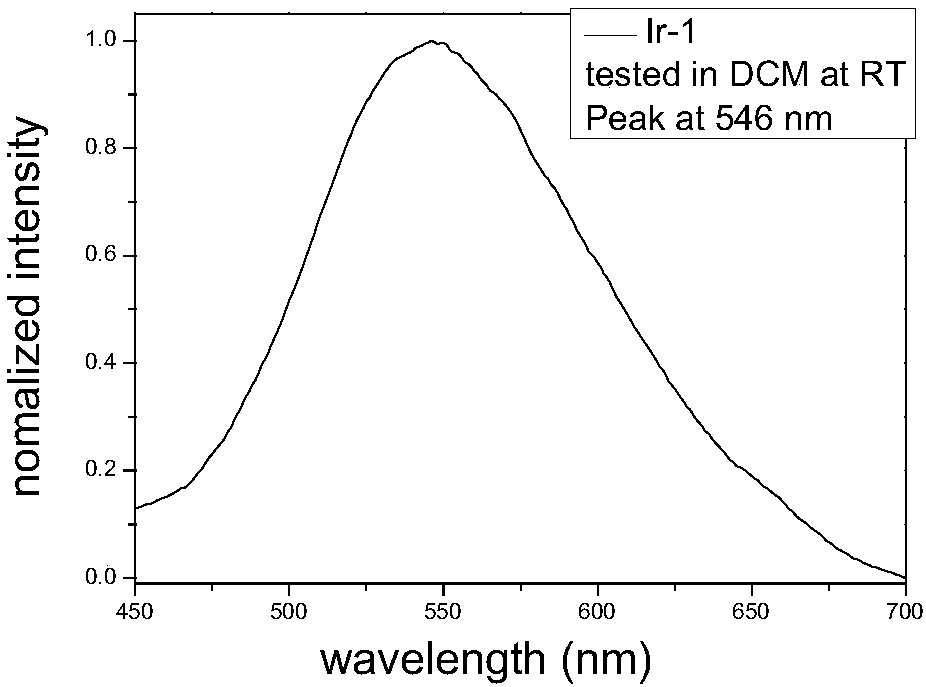
[0117] Synthesis Example 1: Ir-1 synthesis and structure characterization:
[0118]
[0119] Add N-phenylpyrazole, ethylene glycol methyl ether, deionized water and IrCl to the round bottom flask 3 ·3H 2 O, the resulting solution was evacuated three times under nitrogen atmosphere, and then refluxed for another 12 hours. After the reaction, the temperature was returned to room temperature, the solvent was removed by filtration, and the obtained dimer solid was filtered and washed with n-hexane and ether, and then dried in the air.
[0120] Add dimer (0.1g, 0.10mmol, 1.0eq), ligand (0.3g, 1.2mmol, 12.0eq), potassium carbonate (0.3g, 2.2mmol, 22.0eq), glycerol 3mL to the 60mL sealed tube, The temperature was raised to 200° C., the reaction was carried out for 14 hours, and then water was cooled and added. The dichloromethane (DCM) was used for extraction and liquid separation. The organic phase was dried, and PE:EA=10:1 was passed through the column to separate the target points to o...
Synthetic example 2
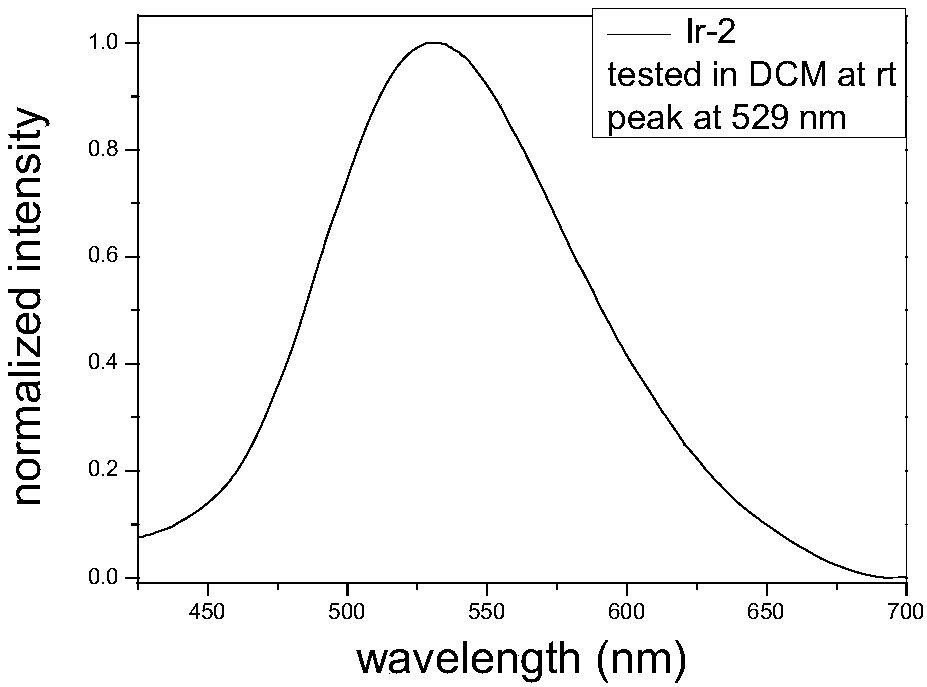
[0124] Synthesis Example 2: Ir-2 synthesis and structure characterization:
[0125]
[0126] Add dimer (0.1g, 0.10mmol, 1.0eq), ligand (0.2g, 0.82mmol, 8.2eq), potassium carbonate (0.3g, 2.2mmol, 22eq), ethylene glycol monomethyl ether 2mL to the 60mL sealed tube, N 2 After bubbling for about 15 minutes, the temperature was raised to 135°C, and the reaction was cooled to room temperature for 20 hours, filtered, and rinsed with 10 mL of water, ethanol, and petroleum ether to obtain 0.09 g of yellow solid with a yield of 62%.
[0127] The luminescence spectrum in dichloromethane solution at room temperature is as follows figure 2 As shown, the main emission peak is at 529nm, which is a green light material.
[0128] 1 H-NMR(300MHz,d 6 -DMSO)δ:5.92-5.95(d,1H),6.03-6.05(d,1H),6.58-6.70(m,3H),6.82-6.91(m,4H),7.49-7.57(m,3H), 7.72 (s, 1H), 7.82-7.85 (d, 1H), 8.00 (d, 1H), 8.05-8.09 (t, 1H), 8.72-8.74 (d, 1H), 8.79-8.80 (d, 1H). ESI MASS: 724.1, [M] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com