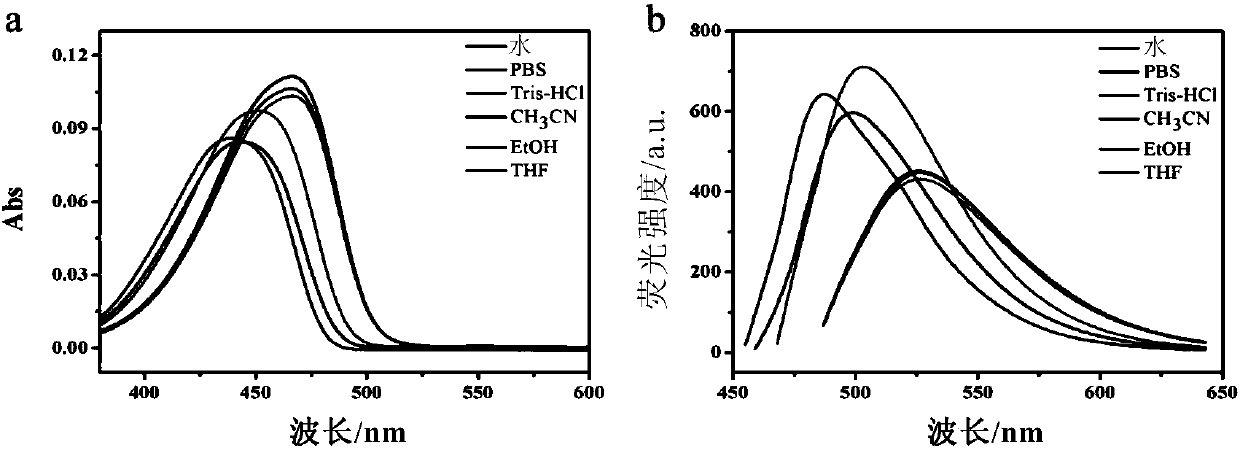
Fluorescent dye based on naphthalimide, and preparation method and application thereof
A naphthalimide and fluorophore technology, applied in the field of naphthalimide-based fluorescent dyes, can solve the problems of poor water solubility, weak fluorescence, prolonged emission wavelength, etc., and achieve the effects of good water solubility and high fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Embodiment 1: the synthesis of compound FM1
[0114]
[0115]M1: Add acenaphthene (3.10g, 20.1mmol) in batches to 20mL of anhydrous DMF, and drop the solution of NBS (3.60g, 20.3mmol) dissolved in 10mL of DMF into the DMF solution of acenaphthene at a constant pressure, keeping the speed at 1 drop / s, the temperature is controlled at room temperature. After stirring overnight, pour into ice water, filter and wash with water to obtain a pale yellow solid. Ethanol was recrystallized to obtain 2.39 g of a light yellow solid, with a yield of 51.0%. Melting point: 55.5-56.6°C.
[0116] 1 H NMR (400MHz, CDCl 3 ): δ7.76(d, J=8.4Hz, 1H), 7.64(d, J=7.6Hz, 1H), 7.53(t, J=7.2Hz, 1H), 7.31(d, J=6.8Hz, 1H ), 7.12 (d, J=7.2Hz, 1H), 3.41 (t, J=7.2Hz, 2H), 3.32 (t, J=7.2Hz, 2H).
[0117] M2: M1 (22.78g, 97.7mmol) was dissolved in 150mL glacial acetic acid in a 500mL two-necked flask, and the temperature was controlled at 10-15°C. Add the mixed solution of 21mL fuming nitric a...
Embodiment 2
[0124] Embodiment 2: the synthesis of compound FM2
[0125]
[0126] M4-2: Dissolve 4-bromo-5-nitro-1,8-naphthalene anhydride (M3, 2.00g, 6.2mmol), 2-(2-aminoethoxy)ethanol (616μL, 6.2mmol) in 20mL In ethanol, reflux 10h, TLC traces until the reaction is complete, and the reaction solution is cooled to room temperature, and the solvent is removed by rotary evaporation, and the silica gel column chromatography (CH 2 Cl 2 / CH 3 OH=200:1, v / v), recrystallized from ethanol to obtain 835 mg of milky white powder solid, yield 33.0%. Melting point: 176.4-176.9°C. 1 H NMR (400MHz, CDCl 3 ): δ8.71(d, J=4.0Hz, 1H), 8.52(d, J=4.0Hz, 1H), 8.21(d, J=4.0Hz, 1H), 7.93(d, J=4.0Hz, 1H ),4.44(t,J=5.6Hz,2H),3.86(t,J=5.6Hz,2H),3.67-3.69(m,2H),3.63-3.65(m,2H),2.08(s,1H) . HRMS (ESI) C 16 h 14 N 2 o 6 Br[M+H] + The theoretical value is 409.0035, and the measured value is 409.0029.
[0127] FM2: Weigh compound M4-2 (200mg, 0.488mmol) and diethanolamine (472μL, 4.88mmol) into a 25mL r...
Embodiment 3
[0128] Embodiment 3: the synthesis of compound FM3
[0129]
[0130] M4-3: Weigh compound M3 (2.00g, 6.21mmol) and 2-aminoethylmorpholine (808mg, 6.21mmol) into a 250mL round bottom flask, add 100mL absolute ethanol to dissolve, heat to 50°C, continue The reaction was stirred for 8h, followed by TLC until the reaction was complete. After the reaction solution was cooled to room temperature, the solvent was removed by rotary evaporation, and silica gel column chromatography (CH 2 Cl 2 / CH 3 OH=200:1, v / v), 1.05 g of milky white powder solid was obtained, and the yield was 38.9%. Melting point: 200.7~201.1℃. 1 H NMR (400MHz, CDCl 3 ): δ8.70(d, J=7.6Hz, 1H), 8.51(d, J=8.0Hz, 1H), 8.22(d, J=8.0Hz, 1H), 7.93(d, J=7.6Hz, 1H ),4.33(t,J=6.2Hz,2H),3.65(br,4H),2.70(t,J=6.2Hz,2H),2.57(br,4H). 13 C NMR (100MHz, CDCl 3): δ162.82, 162.06, 151.28, 135.98, 132.33, 131.23, 130.57, 125.69, 124.17, 123.56, 122.41, 121.21, 66.98, 55.92, 53.79, 37.65. HRMS (ESI) C 18 h 17 N 3 o 5 B...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com