Fluorescent probe used for identifying hydrogen peroxide
A hydrogen peroxide and fluorescent probe technology, applied in the field of chemical analysis and detection, to achieve the effect of wide application range, good selectivity, anti-interference ability, and large Stokes shift
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the synthesis of intermediate product 2
[0028] Compound 1 (234.0 mg, 1.0 mmol) and potassium carbonate (300.1 mg, 2.2 mmol) were stirred in 8.0 mL of acetone at room temperature for 10 minutes. Then 2-(4-(bromomethyl)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (356.2 mg, 1.2 mmol), the resulting mixture was reacted at 70°C for 3h. After the reaction was cooled to room temperature, the reaction mixture was concentrated under reduced pressure to obtain a crude product. Finally, column chromatography was performed using petroleum ether / dichloromethane (5 / 1, v / v) as eluent to obtain compound 3 (197.2 mg, 43.7%) as a yellow solid. 1 H NMR (500MHz, CDCl 3 )δ H 10.26(s, 1H), 7.83(d, J=7.7Hz, 2H), 7.45(d, J=7.7Hz, 2H), 7.01(s, 1H), 6.04, 1.55(s, 2H), 1.36(s , 12H), 1.16(t, J=7.0Hz, 3H), 1.12(t, J=7.1Hz, 3H)
Embodiment 2
[0029] Embodiment 2: the synthesis of probe molecule
[0030] Dissolve compound 2 (90.3 mg, 0.2 mmol) and ethyl cyanoacetate (48.2 mg, 0.4 mmol) in 5.0 mL of ethanol at room temperature, add piperidine (5.0 μL, 0.05 mmol), and stir at room temperature for 5 minutes , and the resulting solution was refluxed for 1 hour. After the reaction, the reaction solution was concentrated under reduced pressure to obtain a crude product. Finally, column chromatography using petroleum ether / ethyl acetate (4 / 1, v / v) as eluent afforded Probe1 as a red solid (46.0 mg, 39.4% yield). HRMS (ESI) m / z: C 22 h 21 N 6 o 5 [M+1]+, 449.1573; 1 H NMR (400MHz, CDCl 3 )δ H 8.72(s, 1H), 7.82(d, J=8.0Hz, 2H), 7.73(s, 1H), 7.42(d, J=8.0Hz, 2H), 5.98(s, 1H), 5.15(s, 2H) , 4.31(q, J=7.1Hz, 2H), 3.31(m, 8H), 1.35(t, J=15.5, 3H), 1.34(s, 12H), 1.21(t, J=7.0Hz, 3H), 1.07 (t, J = 7.1 Hz, 3H). 13 CNMR (100MHz, CDC l 3 )δ C 165.2, 155.9, 147.1, 143.4, 140.0, 135.1, 128.8, 126.1, 118.8, 109.6, 94.4, 9...
Embodiment 3
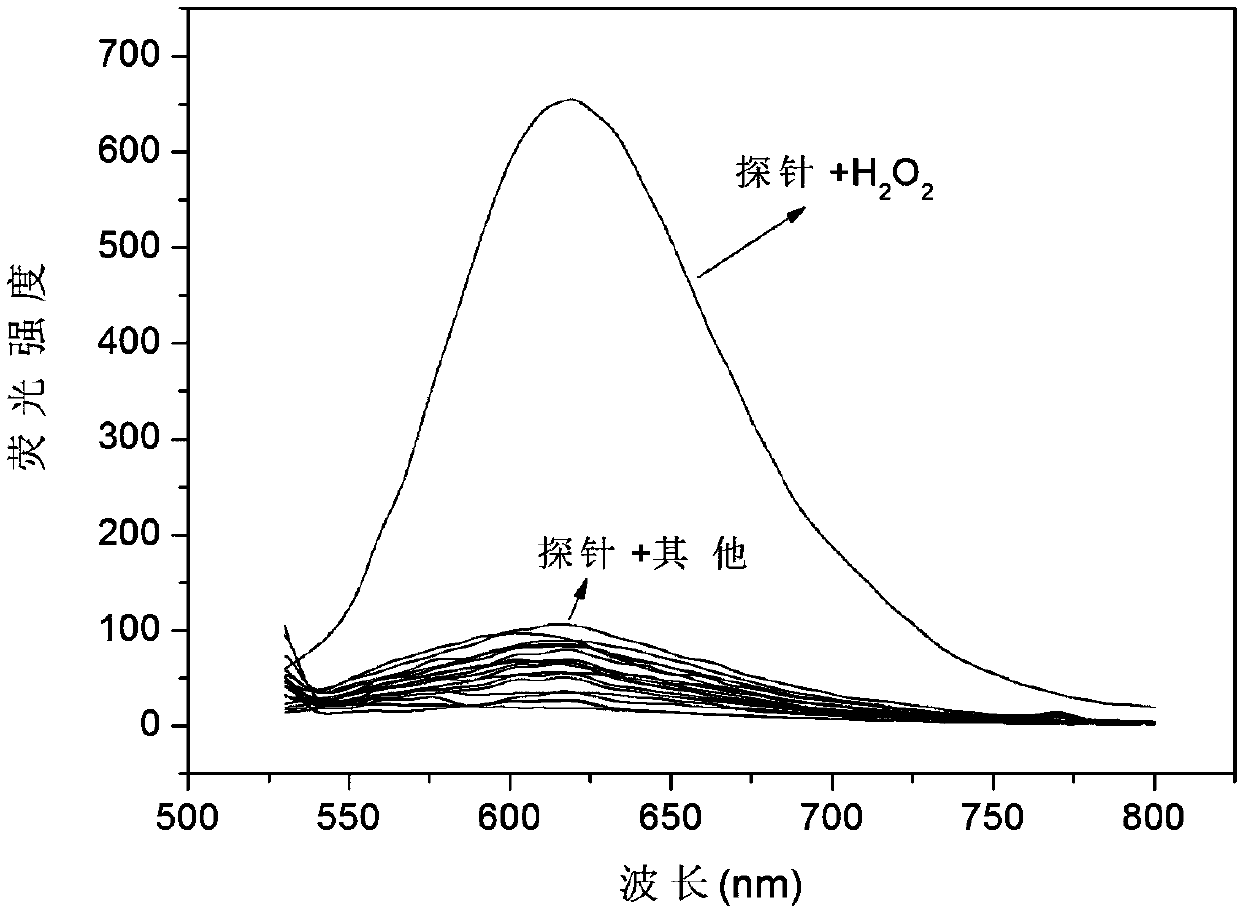
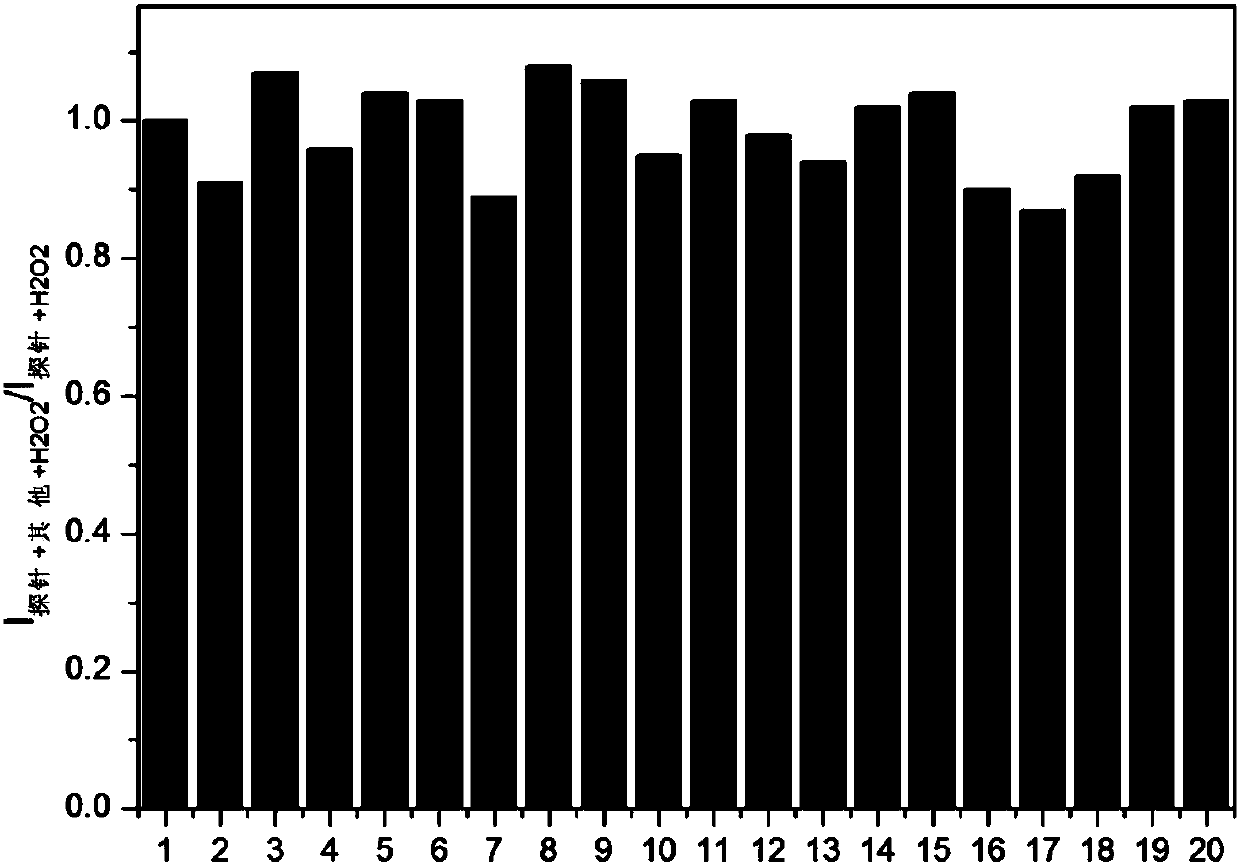
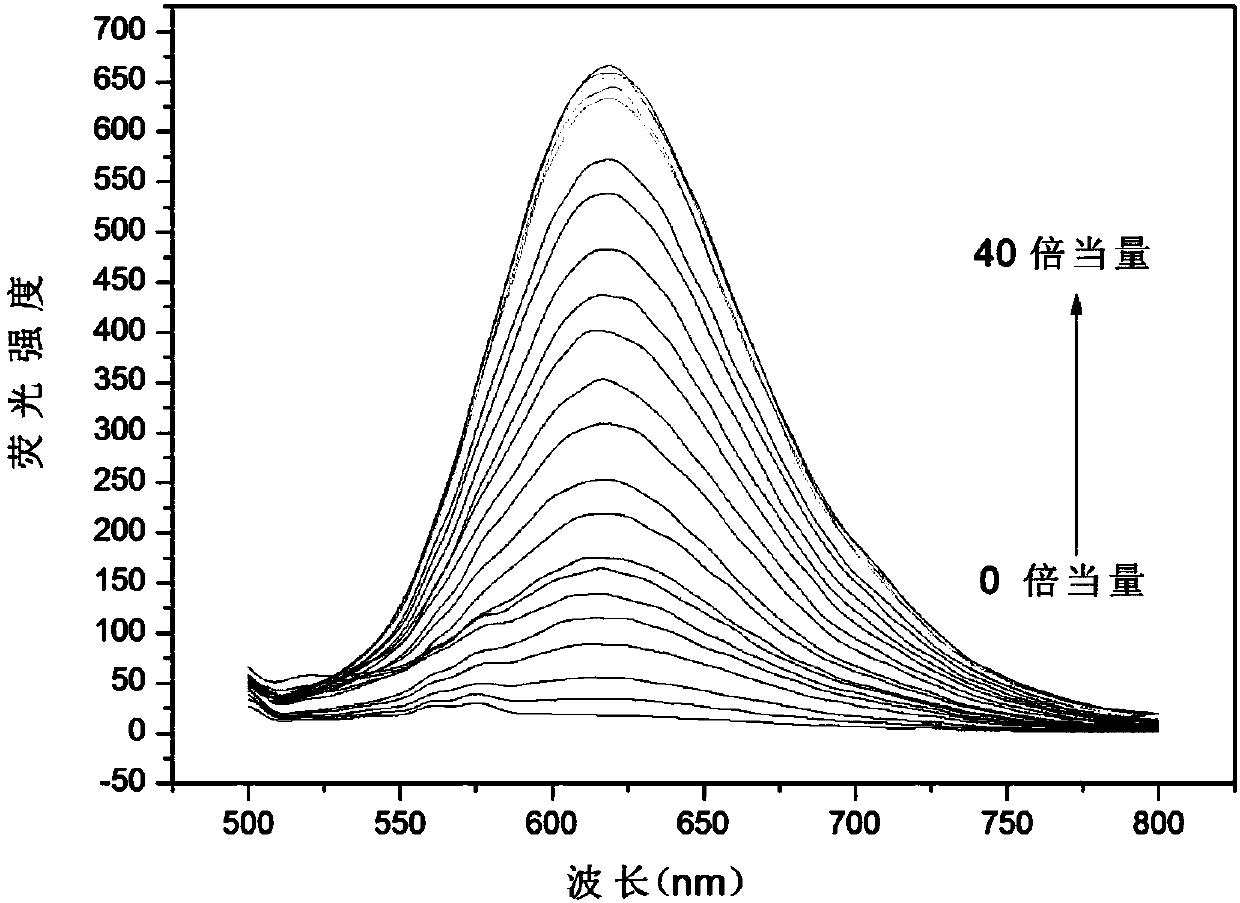
[0031] Embodiment 3: the application of fluorescent probe of the present invention
[0032] The probe molecule was dissolved in PBS (10mM, pH=7.4) / EtOH30% to prepare 10.0×10 -6 mol / L solution, adding various active oxygen species and anions and cations (ROO., NO., TBHP, ClO - , KO 2 , ONOO - ,OH - , I - , F - , PO 4 3- , NO2-, NO 3 - , SO 3 2- , S 2 o 3 2- , SCN - , CN - , CO 3 2- , Fe 2+ , Fe 3+ ) did not cause a significant change in fluorescence, when hydrogen peroxide (H 2 o 2 ) and interfering substances (ROO . , NO . , TBHP, ClO - , KO 2 , ONOO - ,OH - , I - , F - , PO 4 3- , NO2-, NO 3 - , SO 3 2- , S 2 o 3 2- , SCN - , CN - , CO 3 2- , Fe 2+ , Fe 3+ ) coexist, the probe is not affected by interference factors, showing a strong anti-interference ability. The probe molecule and hydrogen peroxide (H 2 o 2 ) response within a certain time and concentration range has a good linear relationship. Probe molecules can react to hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com