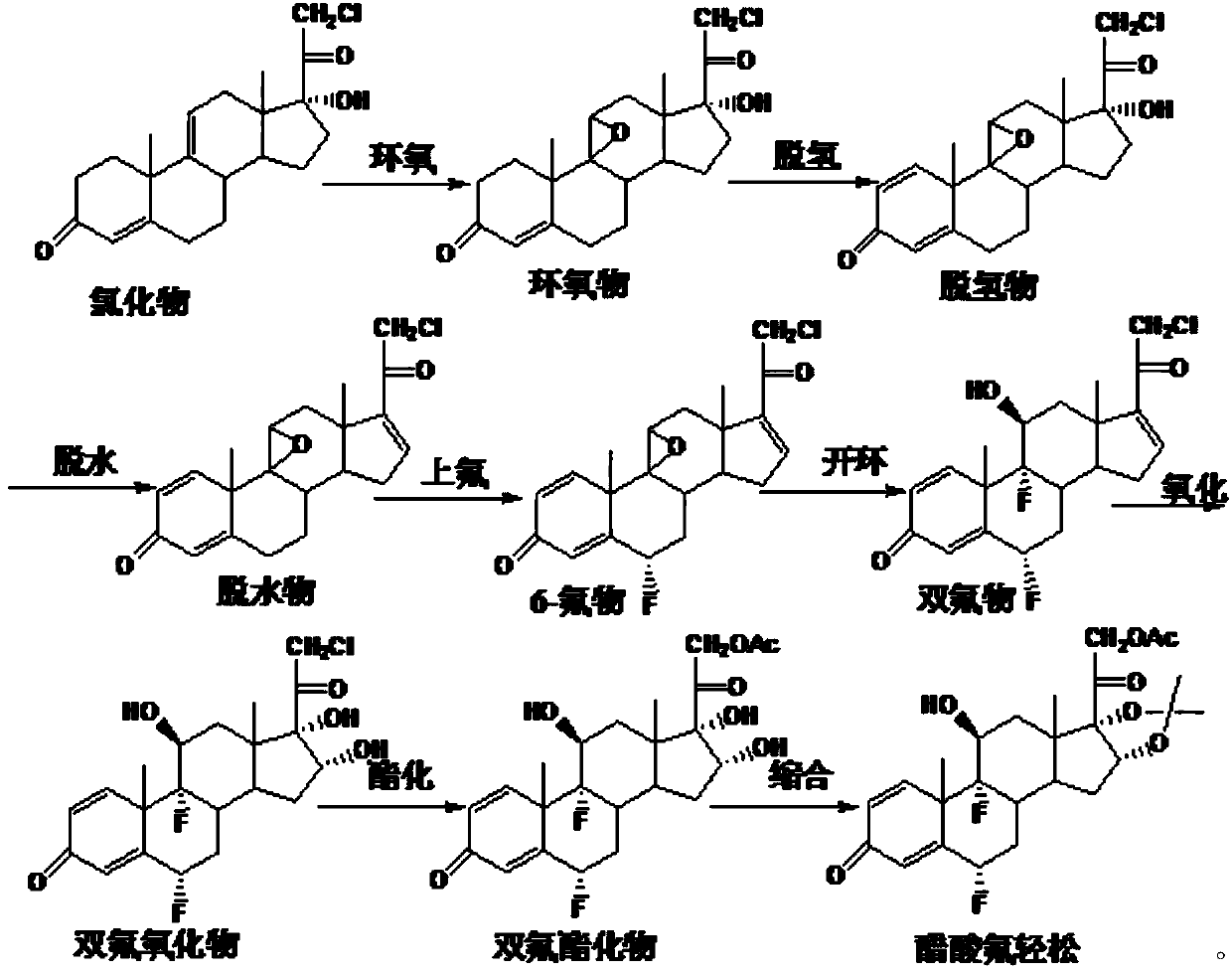
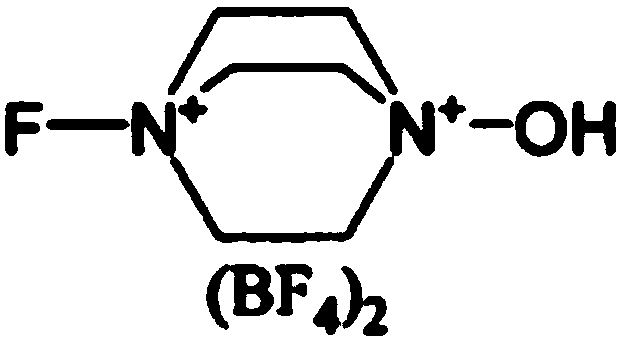
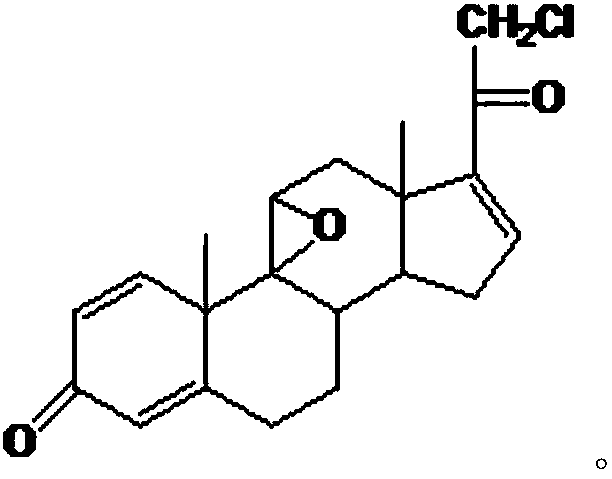
Preparation method of fluocinonide
A technology of fluocinolone acetate and acid catalyst, applied in steroids, fermentation, organic chemistry, etc., can solve problems such as high environmental protection treatment costs of enterprises
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Invention Example 1 Epoxy Reaction
Embodiment 1-1
[0097] Add 10g of chloride, 2ml of perchloric acid, and 120ml of acetone into the reaction flask, stir, cool down to 0°C, add 9g of NBS within 30 minutes, keep the reaction at 5-10°C, and monitor the reaction with thin-layer chromatography Process to the end of the reaction to obtain a halide, add 10% sodium carbonate aqueous solution to neutralize to pH = 6.5, raise the temperature to 20±2°C, add 15ml of 10% sodium hydroxide aqueous solution within 1 hour, and control the temperature at 20-25°C to react. Monitor the progress of the reaction by thin-layer chromatography until the end of the reaction, neutralize with acetic acid to pH=7, concentrate under reduced pressure until there is no acetone smell, dilute into ice water, filter, and dry to obtain 11.2 g of 9,11-epoxide.
Embodiment 1-2
[0099] Add 10g of chloride, 2ml of perchloric acid, and 50ml of tetrahydrofuran into the reaction flask, stir, cool down to 0°C, add 9g of NCS within 30 minutes, keep the reaction at 5-10°C, and monitor the reaction with thin-layer chromatography Process to the end of the reaction, dilute in water, filter to obtain the halide wet product, directly dissolve in 100ml of acetone, heat up to 20±2°C, add 15ml of 10% potassium hydroxide aqueous solution within 1 hour, and control the temperature at 20-25°C for reaction , monitor the progress of the reaction with thin-layer chromatography until the end of the reaction, neutralize the acetic acid to pH=7, concentrate under reduced pressure until there is no acetone smell, dilute into ice water, filter, and dry to obtain 10.9g of 9,11-epoxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com