Preparation method of biosensor for alpha 2,3 saliva acidification glycan detection
A biosensor and sialylation technology, applied in the field of electrochemical detection, can solve problems such as difficulty in generating stable signals, and achieve the effects of facilitating commercialization, improving sensitivity, and increasing conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
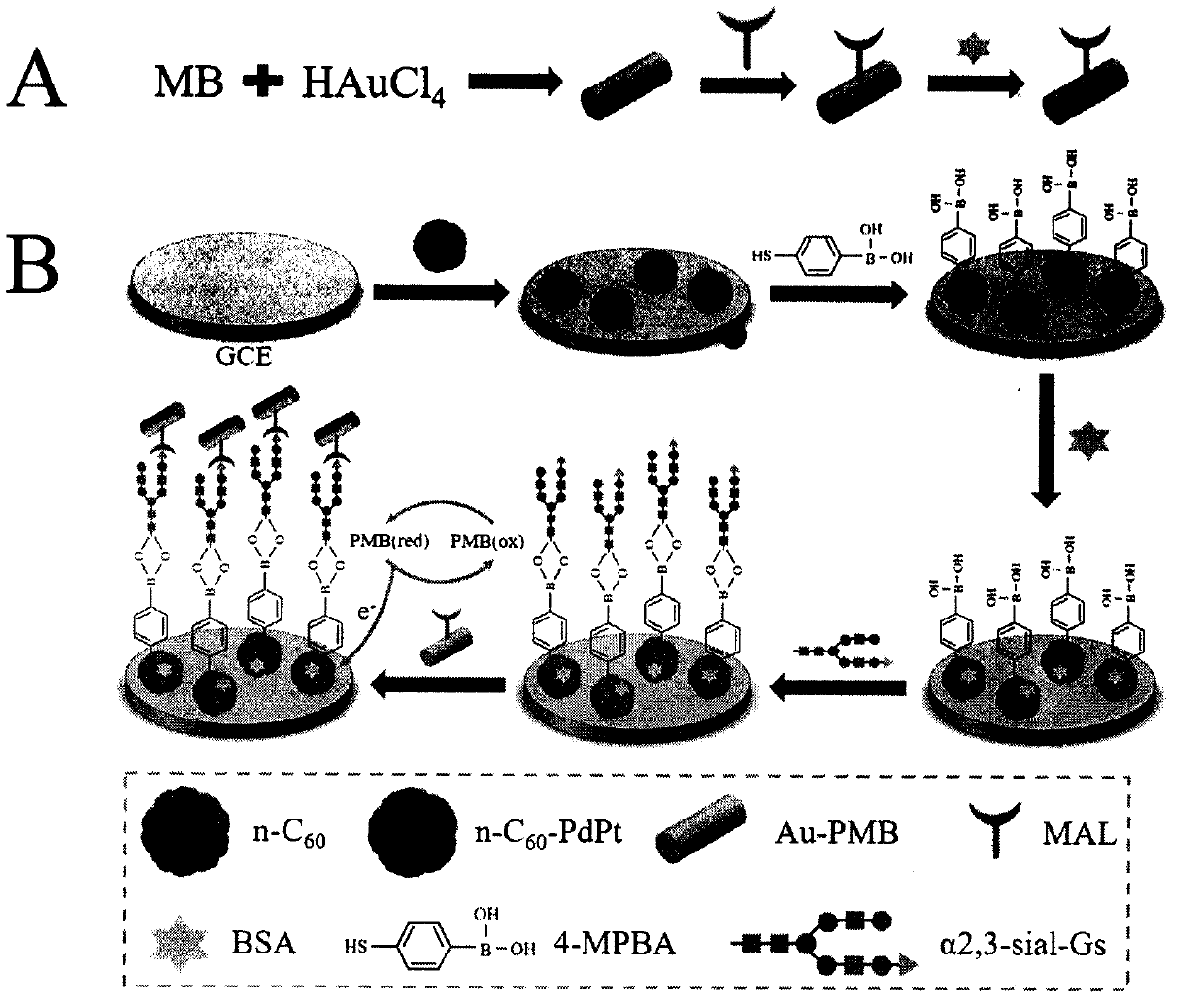
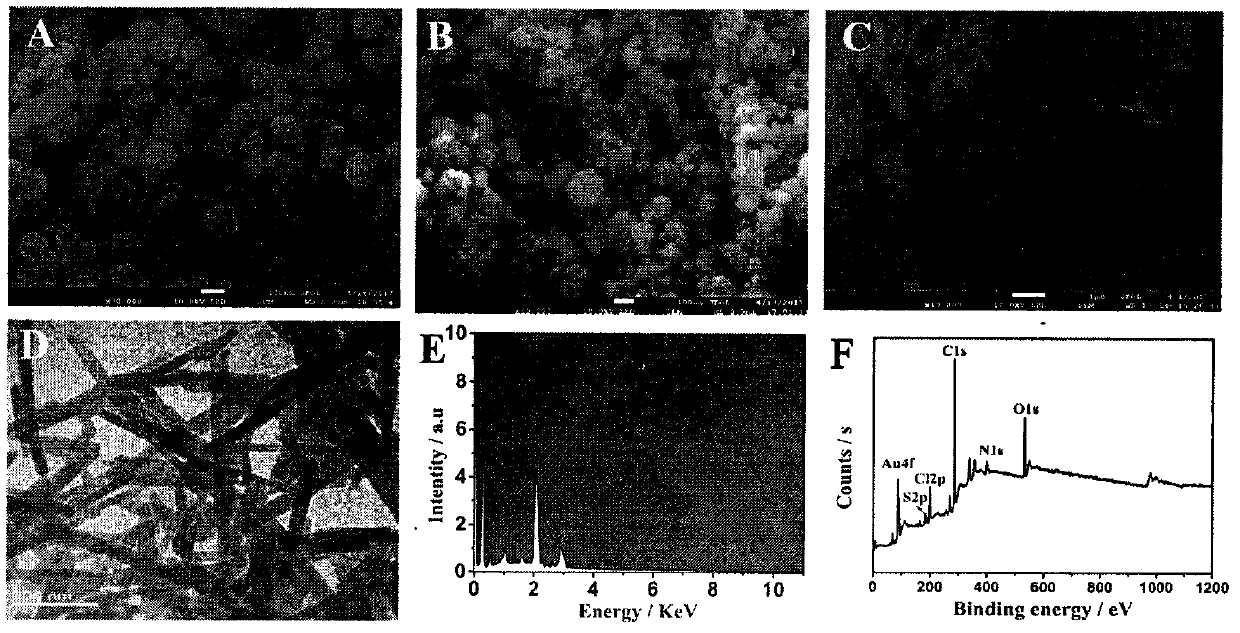
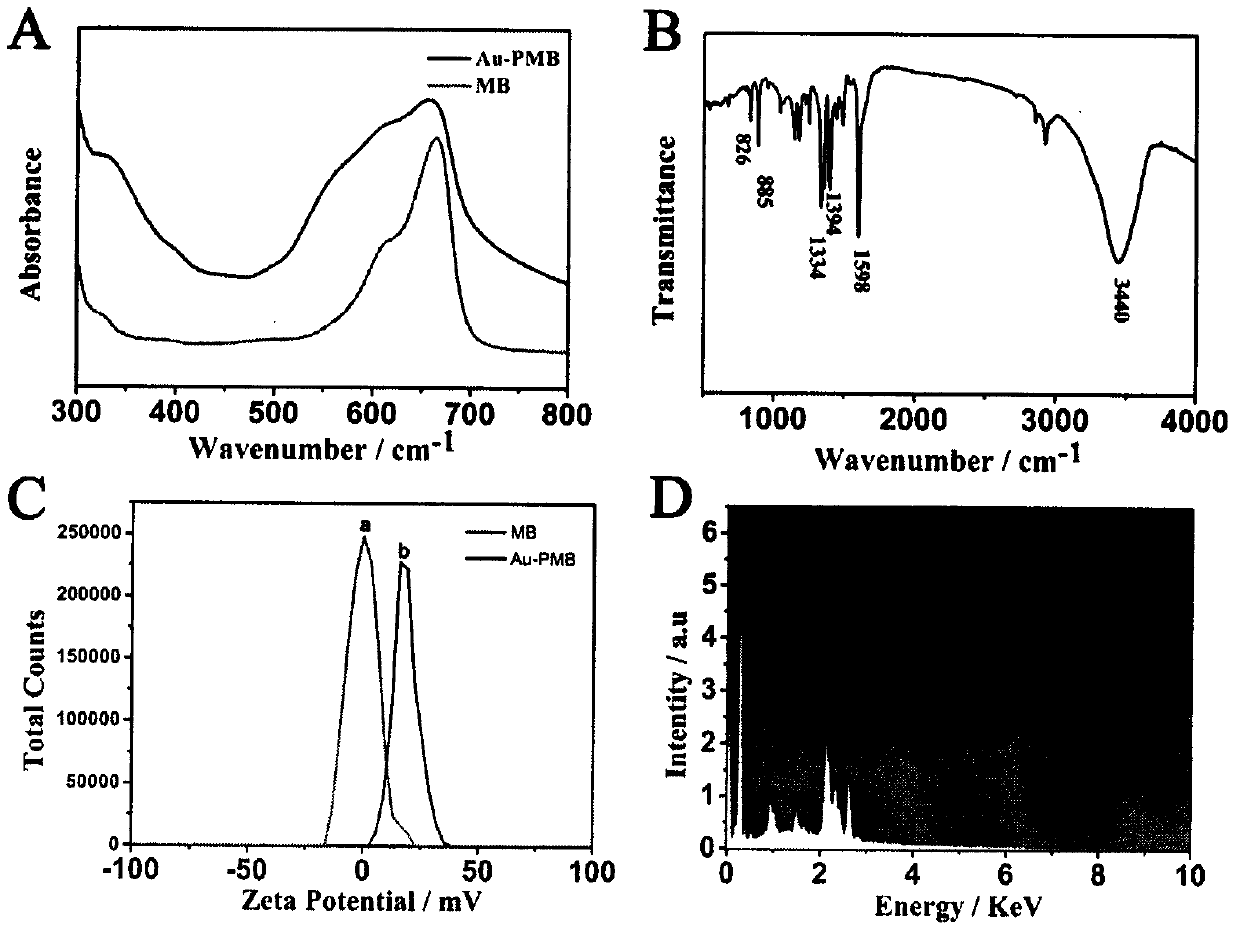
[0039] Step 1. First, disperse 2 mg of n-C60 in 1 mL of 0.1M HCl, then sonicate for 2 hours until n-C60 is uniformly dissolved in the solution. Next, add 2 mL of ultrapure water to the solution. Next, the mixture was stirred and heated to 100°C. After that, 150 mg of AA, 1.17 mg of K 2PtCl 4 and 1.66 mg of Na 2PdCl 4 were added to 1 mL of ultrapure water, respectively. After heating at 100°C for 2.5 hours, the product was cooled to room temperature. Then, the mixture was run at 8000rpm min -1 Centrifuge for 3 minutes and wash three times to remove remaining AA, free Pt and Pd nanoparticles. Finally, dissolve the precipitate in 1 mL of ultrapure water before use for the next step.
[0040] Step 2. First, MB (9.8 mM, 1 mL) was added to HCl (0.1 M, 600 μL) and DTAB (1.14 mM, 6 mL) solution, and stirred thoroughly for about 10 minutes. Subsequently, HAuCl 4 (4%, 200 μL) was added to the compound and stirring was continued at room temperature for about 6 hours. Compound at 80...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com