Taste-masking coating preparation and preparation method thereof
A coating and preparation technology, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. It can solve the problems of dependence on gastric acid environment, release impact, and excessive impact.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
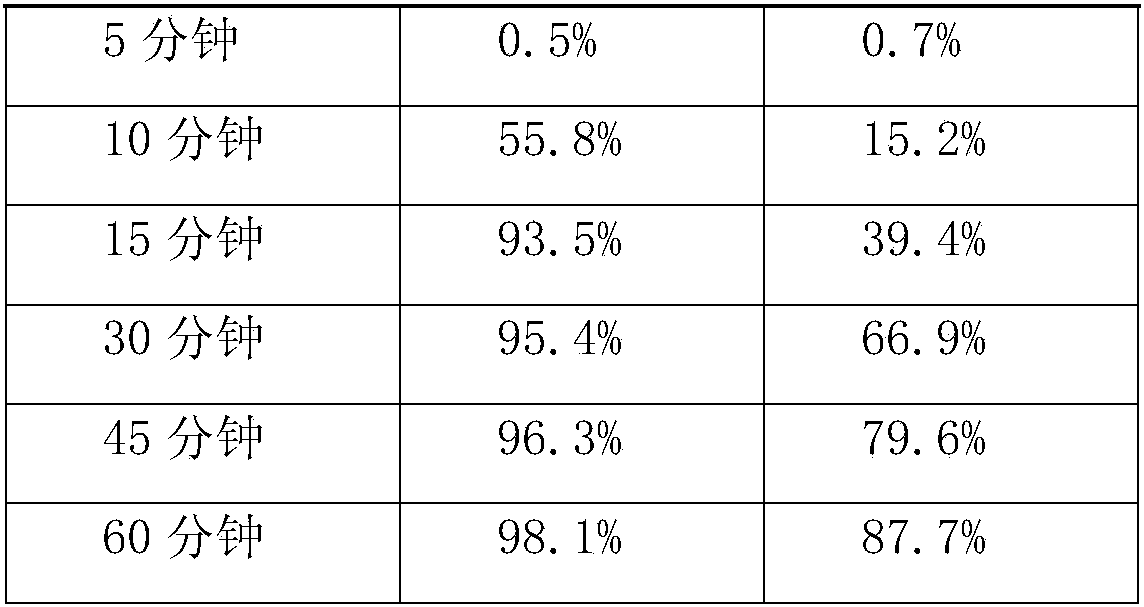
Image
Examples
Embodiment 1
[0035] Embodiment 1. Prednisone taste-masking coated pellets
[0036] The active drug prednisone is loaded in the form of drug-loaded pellets, and the prednisone drug-loaded pellets are sequentially coated with an acidic protective layer, an isolation layer, and a taste-masking layer. The raw materials and dosage of each layer are as follows:
[0037] Drug-loaded pellets:
[0038] Element
Unit dosage
0.5~0.6mm sucrose core
190mg
5mg
Hypromellose E5LV
5mg
Tween 80
1mg
1mg
[0039] Acid protective layer:
[0040] citric acid
5mg
Hypromellose E5LV
1mg
talcum powder
15mg
[0041] Isolation layer:
[0042] Hypromellose E5LV
11.2mg
talcum powder
5mg
[0043] Taste masking layer:
[0044] Eudragit E100
12mg
polyethylene glycol 6000
1.2mg
talcum powder
6mg
[0045] The preparati...
Embodiment 2
[0062] Example 2. Taste-masking coated granules of loratadine
[0063] The loratadine drug powder is used for powder coating directly, and the outer layer of the drug is coated with an acidic protective layer, an isolation layer, and a taste-masking layer in sequence. The raw materials and dosages of each layer are as follows:
[0064] Acid protective layer:
[0065] Element
Unit dosage
5.5mg
Hypromellose E5LV
1.2mg
13mg
[0066] Isolation layer:
[0067] Hypromellose E5LV
12.0mg
5mg
[0068] Taste masking layer:
[0069] Eudragit E100
11.7mg
polyethylene glycol 6000
1.4mg
5.5mg
[0070] The preparation method is:
[0071] 1. Acidic protective film coating:
[0072] Tartaric acid, hypromellose, and stearic acid are prepared into a suspension with water, and fluidized bed coating is selecte...
Embodiment 3
[0079] Example 3. Cefixime taste-masking coated granules
[0080] Use cefixime drug powder to directly carry out powder coating, and coat the acidic protective layer, isolation layer, and taste-masking layer on the outer layer of the drug in sequence. The raw materials and dosage of each layer are as follows:
[0081] Acid protective layer:
[0082] Element
Unit dosage
6.5mg
Hypromellose E5LV
1.2mg
15mg
[0083] Isolation layer:
[0084] Hypromellose E5LV
12.5mg
talcum powder
5mg
[0085] Taste masking layer:
[0086] Eudragit E100
12.5mg
polyethylene glycol 6000
1.1mg
talcum powder
5.5mg
[0087] The preparation method is:
[0088] 1. Acidic protective film coating:
[0089] Malic acid, hypromellose and magnesium stearate are prepared into a suspension with water, and fluidized bed coating is selected, and the dry powder o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com