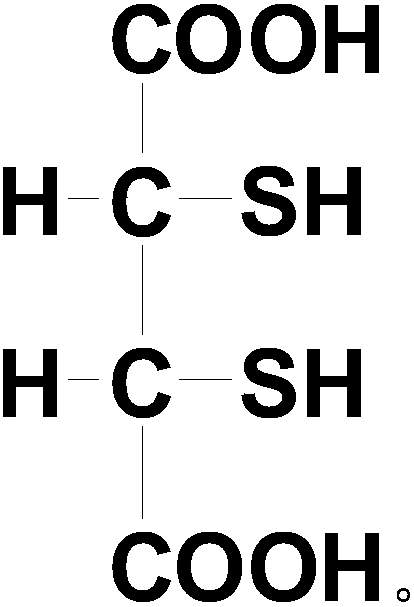
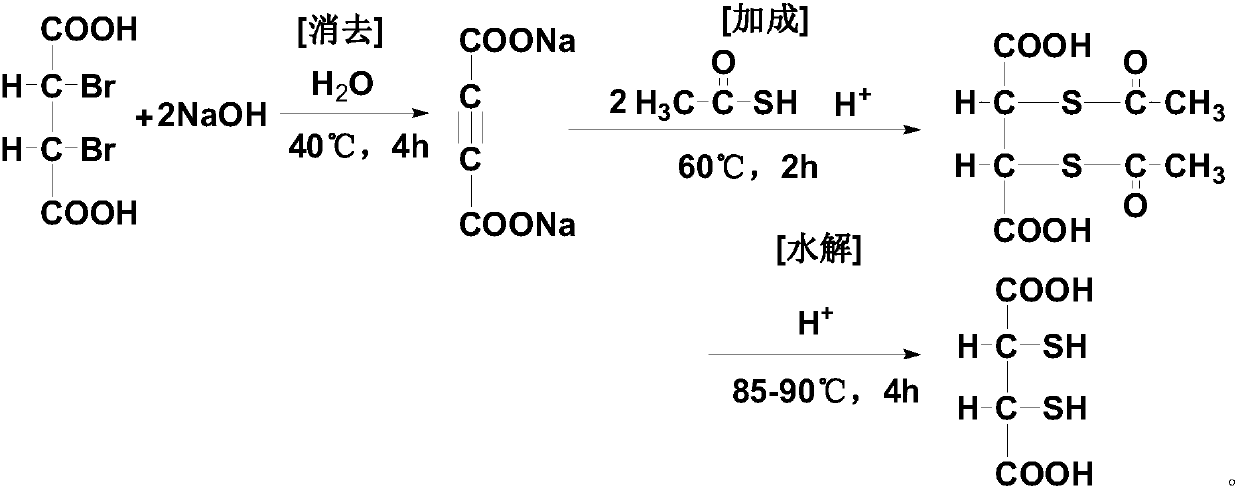
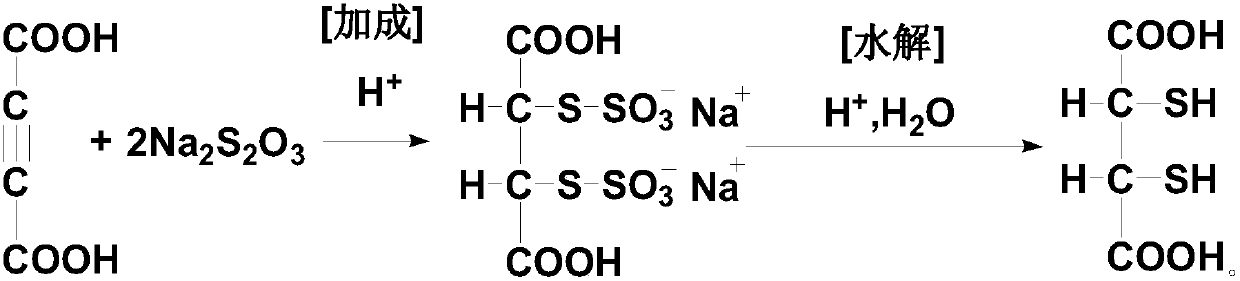
Dimercaptosuccinic acid and salt thereof and preparation method
A technology of dimercaptosuccinate and dimercaptosuccinic acid, which is applied in the field of dimercaptosuccinic acid and its salts and preparation, can solve the problems of high energy consumption, low actual yield, low production capacity, etc., and achieve the preparation method The effect of simple process, short preparation cycle and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] The preparation method of dimercaptosuccinic acid of the present invention is simple in process, and uses a kind of solid ethyl xanthate (sodium salt or potassium salt) with slight odor and low price to replace liquid thioacetic acid with strong odor and unstable quality , and compared with the existing process, one step of elimination reaction is omitted, especially the unsafe addition reaction operation is completely eliminated, which is very beneficial to the future industrial production, the preparation cycle is short, the yield is high, and the cost is low, which is suitable for the promotion of industrial production.
[0074] In a preferred specific embodiment of the present invention, said ethyl xanthate includes one or more of ethyl xanthate metal salts, preferably includes one or more of ethyl xanthate alkali metal salts Various, more preferably sodium ethyl xanthate and / or potassium ethyl xanthate.
[0075] In a preferred embodiment of the present invention, t...
Embodiment 1
[0133] A preparation method for dimercaptosuccinic acid, comprising the steps of:
[0134] 1. Substitution reaction:
[0135] Put 600mL of acetone-water mixed solvent (where the volume ratio of acetone and water is 1:0.8) and 153.1g (content 95.0%, 1.2moL) of potassium ethyl xanthate into a dry and clean 1000mL reaction flask, stir and use The temperature of the water bath was raised to 40-45°C, and 141 g (0.5 moL) of 2,3-dibromosuccinic acid was slowly added after the solution was completely dissolved. After the addition was completed, the temperature was slowly raised to 55° C., and the reaction was continued for 3.0 h, and the end point was determined by HPLC. After the reaction, the reaction solution was slowly cooled to below 15°C, and allowed to stand for 2 hours to allow the precipitation of potassium bromide to fully separate out, and then filtered to remove it. Then the filtrate was vacuum concentrated to 1 / 3 of the original volume and placed in a closed container f...
Embodiment 2
[0147] Under the process of Example 1, the volume ratio of acetone and water in the acetone-water mixed solvent is 1:0.1; the amount of 2,3-dibromosuccinic acid is 169.2g (0.6mol); the substitution reaction is heated to 45 ℃, reacted for 6h; the dilute hydrochloric acid with the mass fraction of 10.0% replaced the aqueous sodium hydroxide solution with the mass fraction of 15.0% in Example 1 as the hydrolysis reaction (nitrogen protection) reagent, and the hydrolysis reaction was heated up to 75 ° C, and the reaction was 6h; acidification temperature Keep at 0°C; other processes and conditions are exactly the same as in Example 1. Finally, 57.7 g of a product of 2,3-dimercaptosuccinic acid was obtained, with a melting point of 191.5°C-192.9°C and a content of 99.60% (HPLC).
[0148] Elemental Analysis Results:
[0149]
[0150] After concentration, decolorization and crystallization from the primary refined mother liquor, 1.98 g of the secondary product was obtained, melti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com