Nanoporous-structure oxygen evolution catalyst with iridium oxide enriched on surface and preparation method for oxygen evolution catalyst
A nanoporous, surface oxidation technology, applied in the field of electrochemistry, can solve the problems of low conductivity and high IrO2 usage, and achieve the effects of low cost, controllable preparation process conditions and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] A specific scheme of a preparation method of a nanoporous structure oxygen evolution catalyst enriched in iridium oxide on the surface comprises the following steps:
[0038] Step 1: adding chloroiridic acid hydrate and cobalt chloride hydrate precursors to the NaOH solution to obtain the first solution; the ratio of substances n(Ir):n(Co)=1~3:1~2, n(NaOH)=20n(Ir+Co);
[0039] Step 2: The first solution described in step 1 is reacted in a water bath at 80°C until the solvent is evaporated to dryness to obtain the first product;
[0040] Step 3: burning the first product described in step 2 in an oxygen atmosphere for 1 hour at a burning temperature of 450° C., and cooling to room temperature to obtain a second product;
[0041] Step 4: The second product described in step 3 was dissolved in 5M HNO 3 stirring for 24 hours for acid etching, finally washing, suction filtration, and drying to obtain a nanoporous oxygen evolution catalyst with surface iridium oxide enriche...
Embodiment 1
[0043] 1) put H 2 IrCl 6 ·6H 2 O and CoCl 2 ·6H 2 The O precursor is added to the NaOH solution, the substance ratio n(Ir):n(Co)=1:1, n(NaOH)=20n(Ir+Co), and the first solution is obtained.
[0044]2) The first solution described in 1) was reacted in a water bath at 80°C until the solvent was evaporated to dryness to obtain the first product.
[0045] 3) The first product described in 2) was burned in an oxygen atmosphere for 1 hour at a temperature of 450° C., and cooled to room temperature to obtain a second product.
[0046] 4) 3) the second product in 5M HNO 3 stirring for 24 hours for acid etching, finally washing, suction filtration, and drying to obtain a nanoporous oxygen evolution catalyst with surface iridium oxide enriched.
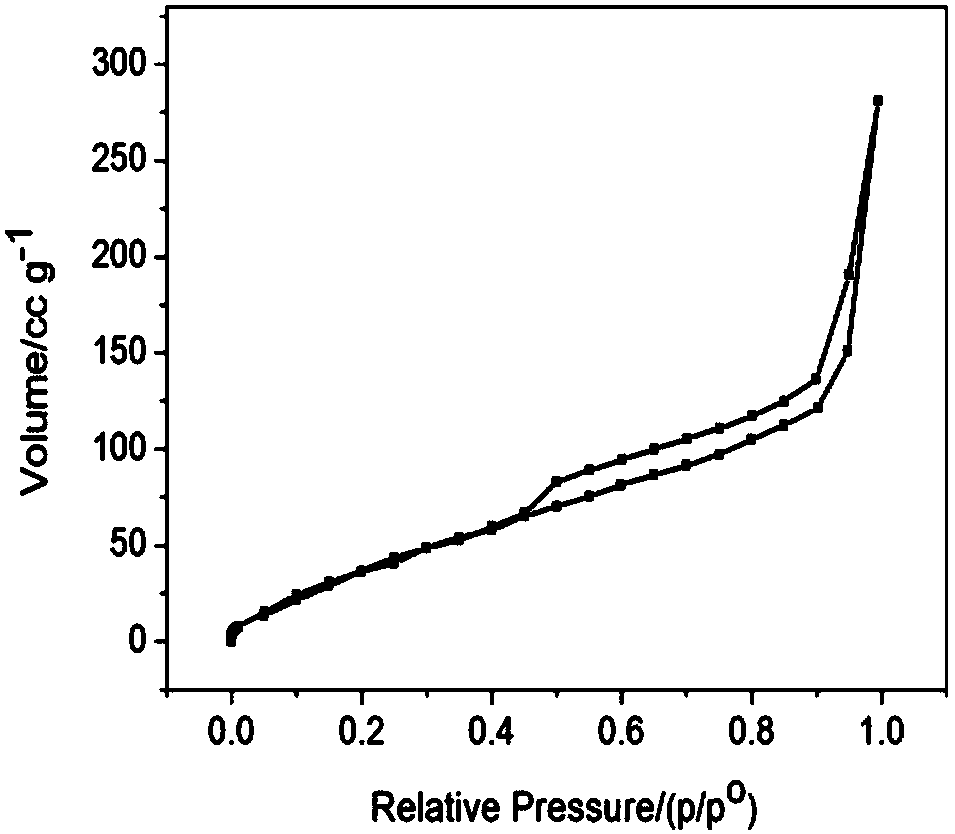
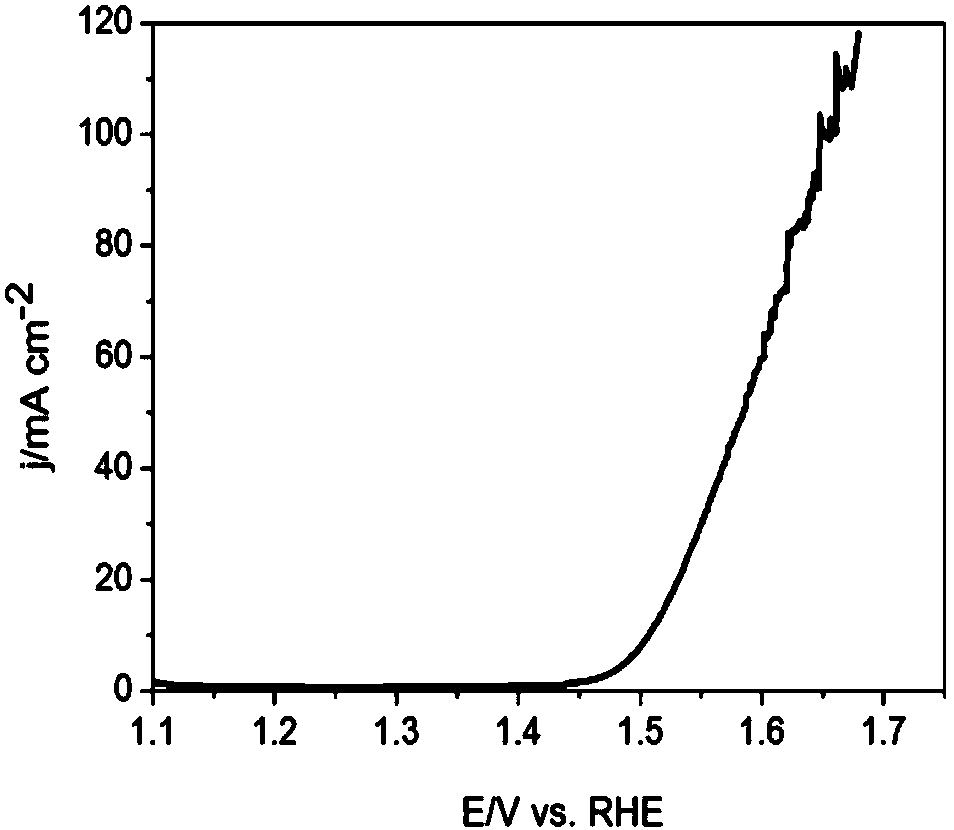
[0047] 5) ultrasonically disperse the obtained surface iridium oxide-enriched nanoporous structure oxygen evolution catalyst in ethanol to obtain a suspension; apply the obtained suspension to a copper grid and dry it, then scan it with a...
Embodiment 2
[0050] 1) put H 2 IrCl 6 ·6H 2 O and CoCl 2 ·6H 2 The O precursor is added to the NaOH solution, the substance ratio n(Ir):n(Co)=1:2, n(NaOH)=20n(Ir+Co), and the first solution is obtained.
[0051] 2) The first solution described in 1) was reacted in a water bath at 80°C until the solvent was evaporated to dryness to obtain the first product.
[0052] 3) The first product described in 2) was burned in an oxygen atmosphere for 1 hour at a temperature of 450° C., and cooled to room temperature to obtain a second product.
[0053] 4) 3) the second product in 5M HNO 3 stirring for 24 hours for acid etching, finally washing, suction filtration, and drying to obtain the nanoporous structure oxygen evolution catalyst enriched in iridium oxide on the surface.
[0054] 5) ultrasonically disperse the obtained surface iridium oxide-enriched nanoporous structure oxygen evolution catalyst in ethanol to obtain a suspension; apply the obtained suspension to a copper grid and dry it, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com