Chiral isoxadifen-ethyl compound and preparation method and application thereof
A technology of ethyl isoxadifen and ethyl stilbene ketonate, which is applied in the field of ethyl isoxadifen, can solve the problems of unfavorable industrial application, waste, and low product yield, and achieve outstanding results Substantial characteristics, mild reaction conditions, and the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
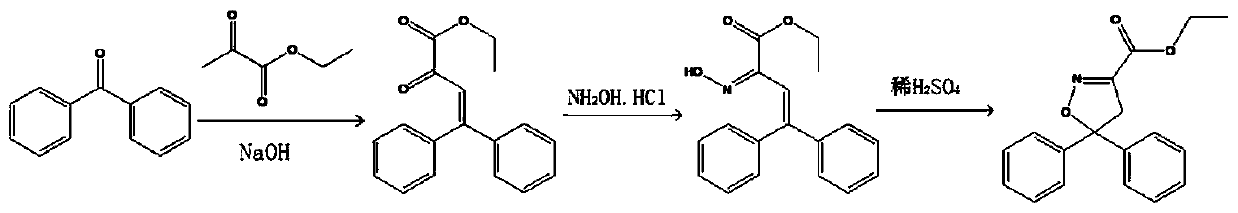
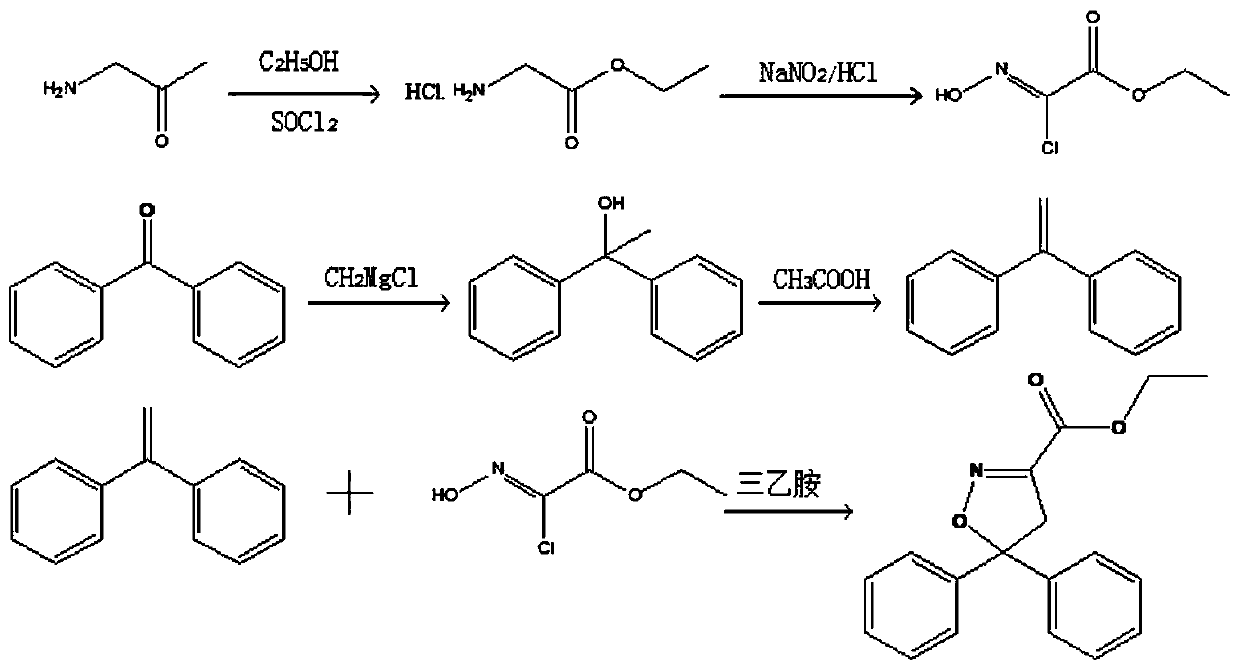
[0031] Such as figure 1 As shown, this embodiment provides a preparation method of chiral isoxadifen ethyl ester compound, which comprises the following steps:
[0032] The cross-aldol condensation reaction first weighs benzophenone and ethyl pyruvate according to the molar ratio of 1.2:1.0, and then takes the benzophenone and the ethyl pyruvate mass sum of 3 times the mass of the hydroxide sodium solution, adding the benzophenone into the sodium hydroxide solution, then dropwise adding the ethyl pyruvate, reacting at 50-90°C for 3 hours, then removing the solvent, and recrystallizing with 60% ethanol solution, Filtrate to obtain ethyl distyrene ketoacetate;
[0033] Oximation reaction Mix the ethyl distyrene ketovate and hydroxylamine hydrochloride uniformly according to the molar ratio of 1:0.8, react at 50-70°C for 0.3-0.8h, then react at room temperature for 0.2-0.7h, and filter , dry treatment, obtain ethyl distyryl formate ketone oxime;
[0034] Addition and ring clos...
Embodiment 2
[0037] Such as figure 1 As shown, this embodiment provides a preparation method of chiral isoxadifen ethyl ester compound, which comprises the following steps:
[0038] The cross-aldol condensation reaction first weighs benzophenone and ethyl pyruvate according to the molar ratio of 1.5:1.0, and then takes 5 times the mass sum of the benzophenone and ethyl pyruvate. Potassium solution, add the benzophenone into the sodium hydroxide solution, then drop into the ethyl pyruvate, react at 50-90°C for 3h, then remove the solvent, recrystallize with 70% ethanol solution, Filtrate to obtain ethyl distyrene ketoacetate;
[0039] The oximation reaction mixes the ethyl distyrene ketovate and hydroxylamine hydrochloride uniformly according to the molar ratio of 1:1.2, reacts at 50-70°C for 0.3-0.8h, and then reacts at room temperature for 0.2-0.7h, After filtration and drying treatment, ethyl distyryl formate ketone oxime was obtained;
[0040] Addition and ring closure reaction Mix t...
Embodiment 3
[0043] Such as figure 1 As shown, this embodiment provides a preparation method of chiral isoxadifen ethyl ester compound, which comprises the following steps:
[0044]The cross-aldol condensation reaction first weighs benzophenone and ethyl pyruvate according to the molar ratio of 1.3:1.0, and then takes 4 times the mass sum of the benzophenone and ethyl pyruvate. sodium solution, adding the benzophenone into the sodium hydroxide solution, then dropwise adding the ethyl pyruvate, reacting at 50-90°C for 3 hours, then removing the solvent, and recrystallizing with 80% ethanol solution, Filtrate to obtain ethyl distyrene ketoacetate;
[0045] The oximation reaction mixes the ethyl distyrene ketovate and hydroxylamine hydrochloride uniformly according to the molar ratio of 1:1.1, reacts at 50-70°C for 0.3-0.8h, and then reacts at room temperature for 0.2-0.7h, After filtration and drying treatment, ethyl distyryl formate ketone oxime was obtained;
[0046] Addition and ring c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com