Preparation and inclusion method of albendazole inclusion complex tablets
A technology of albendazole and inclusion complex, applied in the field of pharmaceutical preparations, can solve the problems of low bioavailability, poor solubility of albendazole and the like, and achieve the effects of simple preparation process, improved in-vitro performance, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] prescription:
[0030] Albendazole
20g
85.4g
100g
60g
5g
[0031] Made in 1000 pieces.
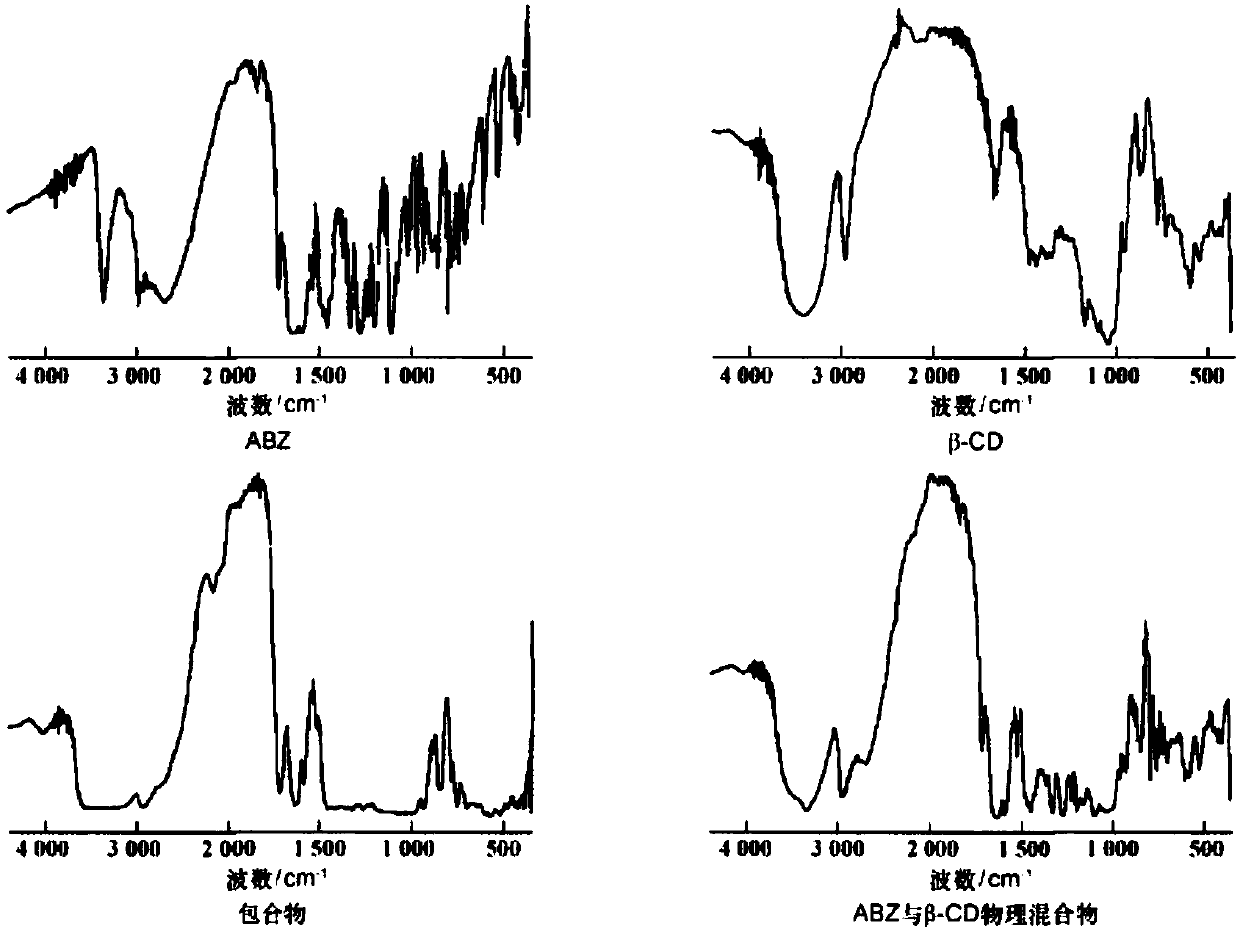
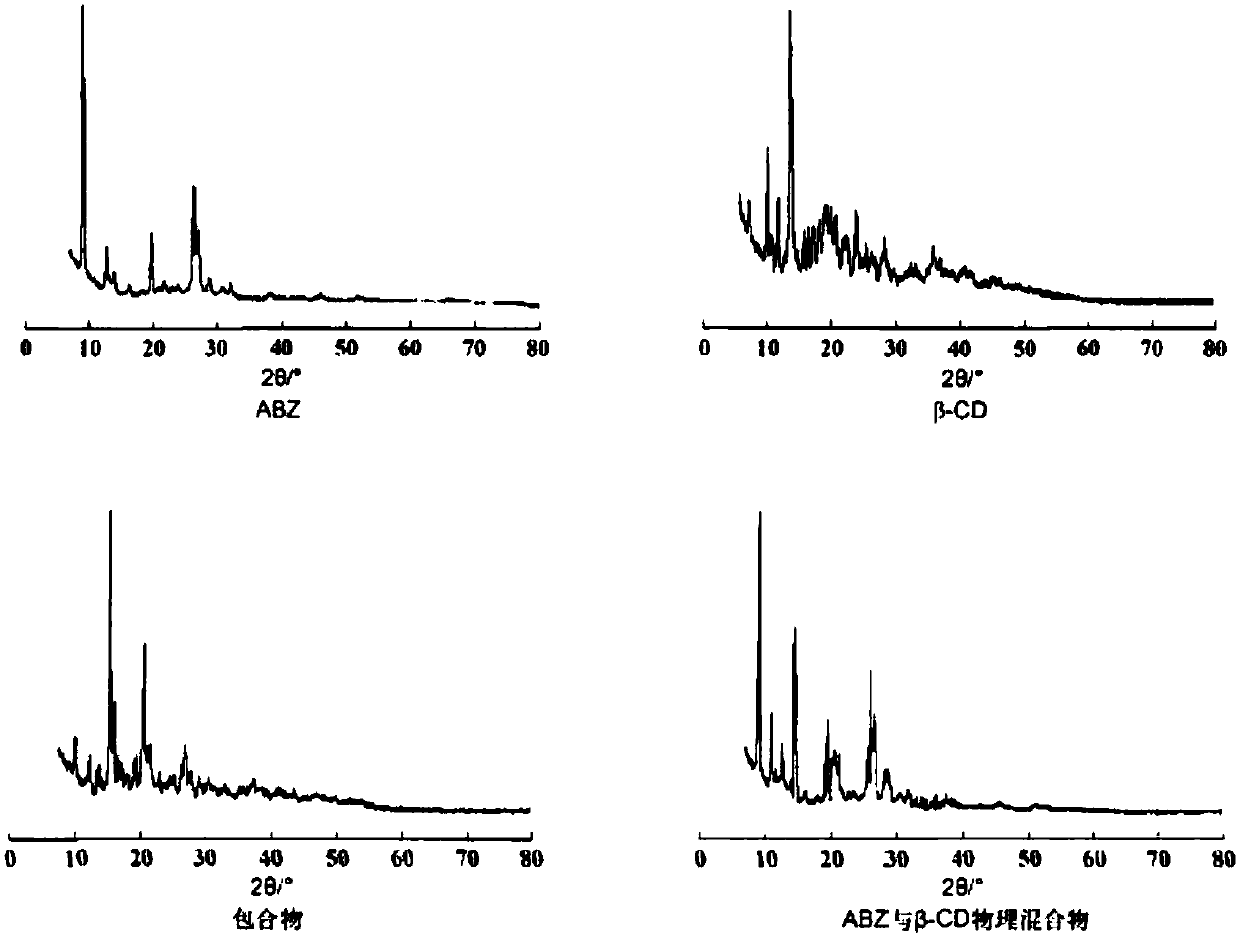
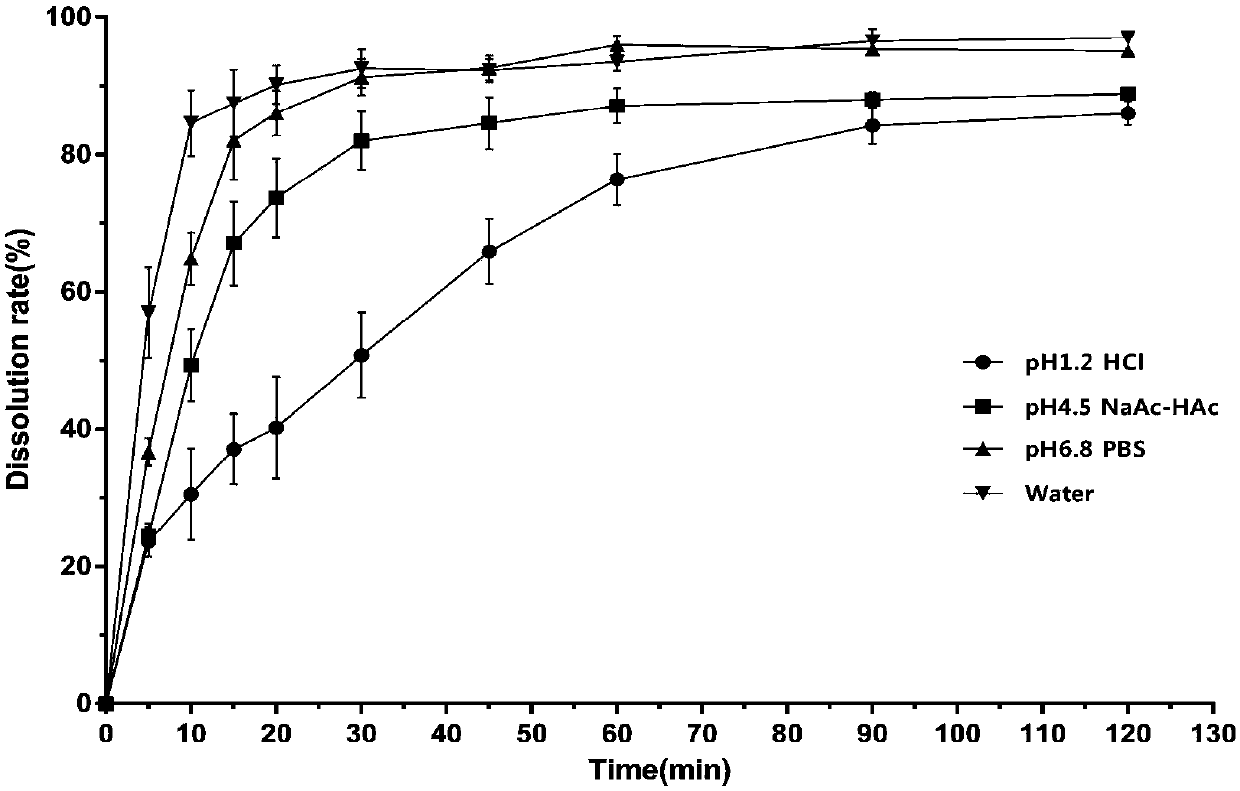
[0032] Preparation method: After dissolving albendazole in formic acid, add it to the mixed solution of acetone: ethanol = 1:1. Dissolve β-cyclodextrin in water and add it dropwise to the mixed solution of albendazole, stir at 55°C for 6h, then put it in the refrigerator for 1h, filter, wash the solid with pure water, and dry it in a blast oven at 50°C Dry to obtain clathrate. Albendazole clathrate and filler microcrystalline cellulose, disintegrating agent croscarmellose sodium and lubricant magnesium stearate are mixed again, and then directly powdered into tablets with a tablet machine. The weight of the dose should be within 3~4kg, and the disintegration time should be within 1~2min.
Embodiment 2
[0034] prescription:
[0035] Albendazole
20g
β-cyclodextrin
170.8g
100g
60g
5g
[0036] Made in 1000 pieces.
[0037] Preparation method: After dissolving albendazole in formic acid, add it to the mixed solution of acetone: ethanol = 1:1. Dissolve β-cyclodextrin in water and add it dropwise to the mixed solution of albendazole, stir at 55°C for 6h, then put it in the refrigerator for 1h, filter, wash the solid with pure water, and dry it in a blast oven at 50°C Dry to obtain clathrate. Albendazole clathrate and filler microcrystalline cellulose, disintegrating agent croscarmellose sodium and lubricant magnesium stearate are mixed again, and then directly powdered into tablets with a tablet machine. The weight of the dose should be within 3~4kg, and the disintegration time should be within 1~2min.
Embodiment 3
[0039] prescription:
[0040] Albendazole
20g
β-cyclodextrin
256.2g
100g
60g
Magnesium stearate
5g
[0041] Made in 1000 pieces.
[0042] Preparation method: After dissolving albendazole in formic acid, add it to the mixed solution of acetone: ethanol = 1:1. Dissolve β-cyclodextrin in water and add it dropwise to the mixed solution of albendazole, stir at 55°C for 6h, then put it in the refrigerator for 1h, filter, wash the solid with pure water, and dry it in a blast oven at 50°C Dry to obtain clathrate. Albendazole clathrate and filler microcrystalline cellulose, disintegrating agent croscarmellose sodium and lubricant magnesium stearate are mixed again, and then directly powdered into tablets with a tablet machine. The weight of the dose should be within 3~4kg, and the disintegration time should be within 1~2min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com