Scar removing composition and preparation method thereof
A composition and scar removal technology, applied in the directions of drug combinations, active ingredients of hydroxyl compounds, pharmaceutical formulations, etc., can solve problems such as the inability to meet the needs of the market, and achieve the effects of eliminating various scars, reducing pigment deposition, and promoting degradation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 composite bioactive factor nano liposome
[0027]
[0028]
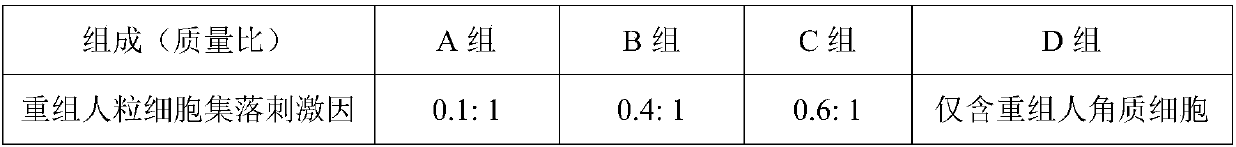
[0029] The preparation of the complex bioactive factor nanoliposome described in group A of the present invention comprises the following steps:
[0030] (1) Weigh 0.2g of phosphatidylcholine, 0.08g of cholesterol, and 0.04g of distearoylphosphatidylethanolamine, mix them, dissolve in 50mL of ethanol, and sonicate for 5min;
[0031] (2) Rotate evaporation at 28°C and 0.1MPa to remove ethanol, let it stand for 30min, add 100mL pH of 7.4 phosphate buffer solution containing 0.5% (m / v) complex biologically active factors, and place in a water bath at 35°C Medium shaking hydration reaction for about 1 hour to obtain liposome suspension;
[0032] (3) Use a syringe filter to adjust the particle size of the liposome suspension to 150nm to obtain nanoliposomes of complex bioactive factors.
[0033] For the preparation of complex biologically active factor nanoliposomes in groups...
Embodiment 2-4 and comparative example 1-4
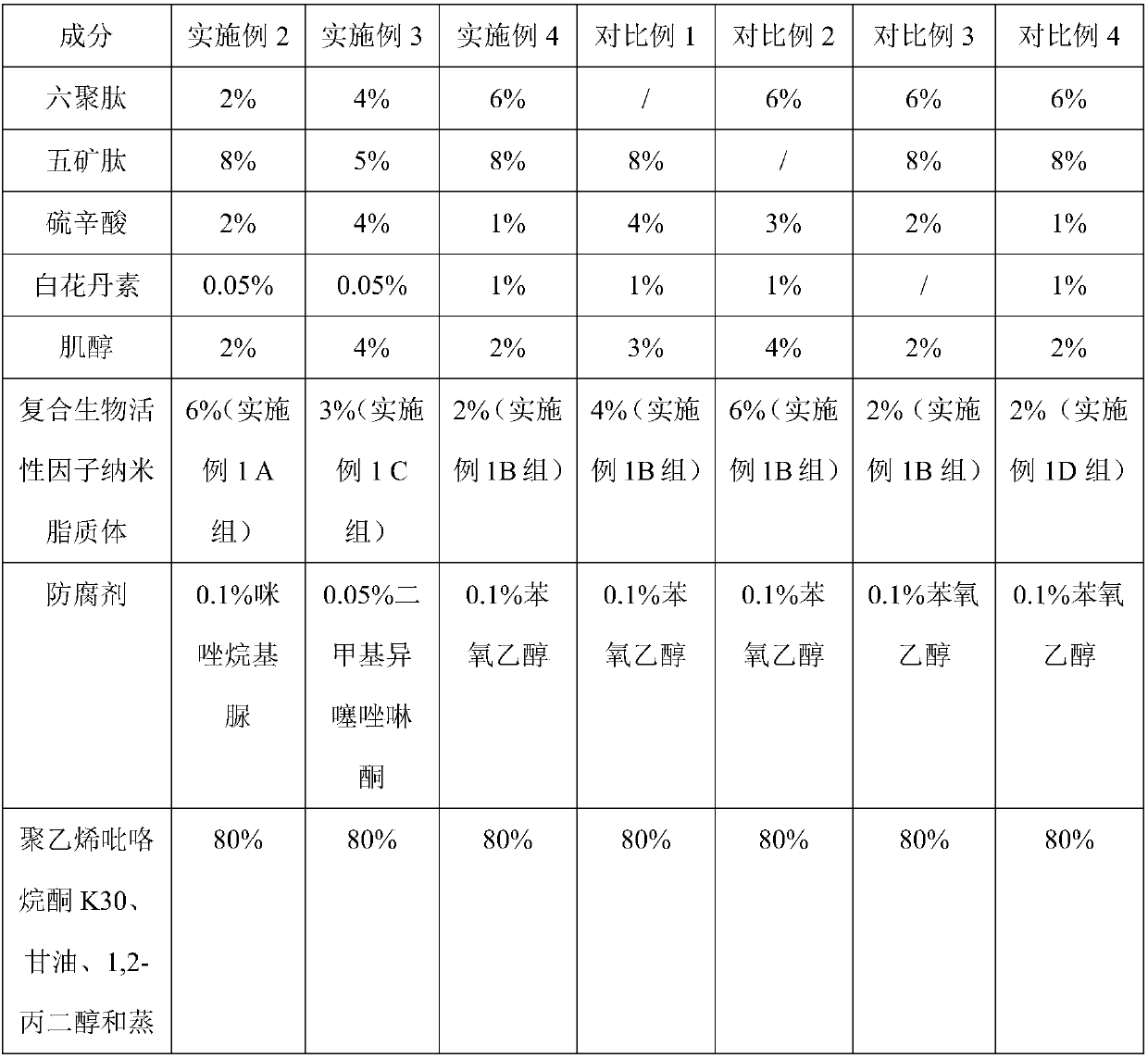
[0034] Preparation of Example 2-4 and Comparative Example 1-4 Composition for Dispelling Scars
[0035] The composition of embodiment 2-4 of the present invention and comparative example 1-4 scar removal composition is shown in the table below:
[0036]
[0037]
Embodiment 2
[0038] The preparation of embodiment 2 scar removal compositions:
[0039] Dissolve polyvinylpyrrolidone K30 in distilled water, add glycerin and 1,2-propanediol, and stir evenly to make a matrix; add lipoic acid, hexamerpeptide, minetal peptide, plumbagin, and inositol in sequence, stir well, and finally Add composite bioactive factor nanoliposome and imidazolidinyl urea, vortex for 4 minutes, and obtain.
[0040] For the preparation of the scar-removing compositions of Examples 3 and 4 and Comparative Examples 1-4, refer to the above-mentioned method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com