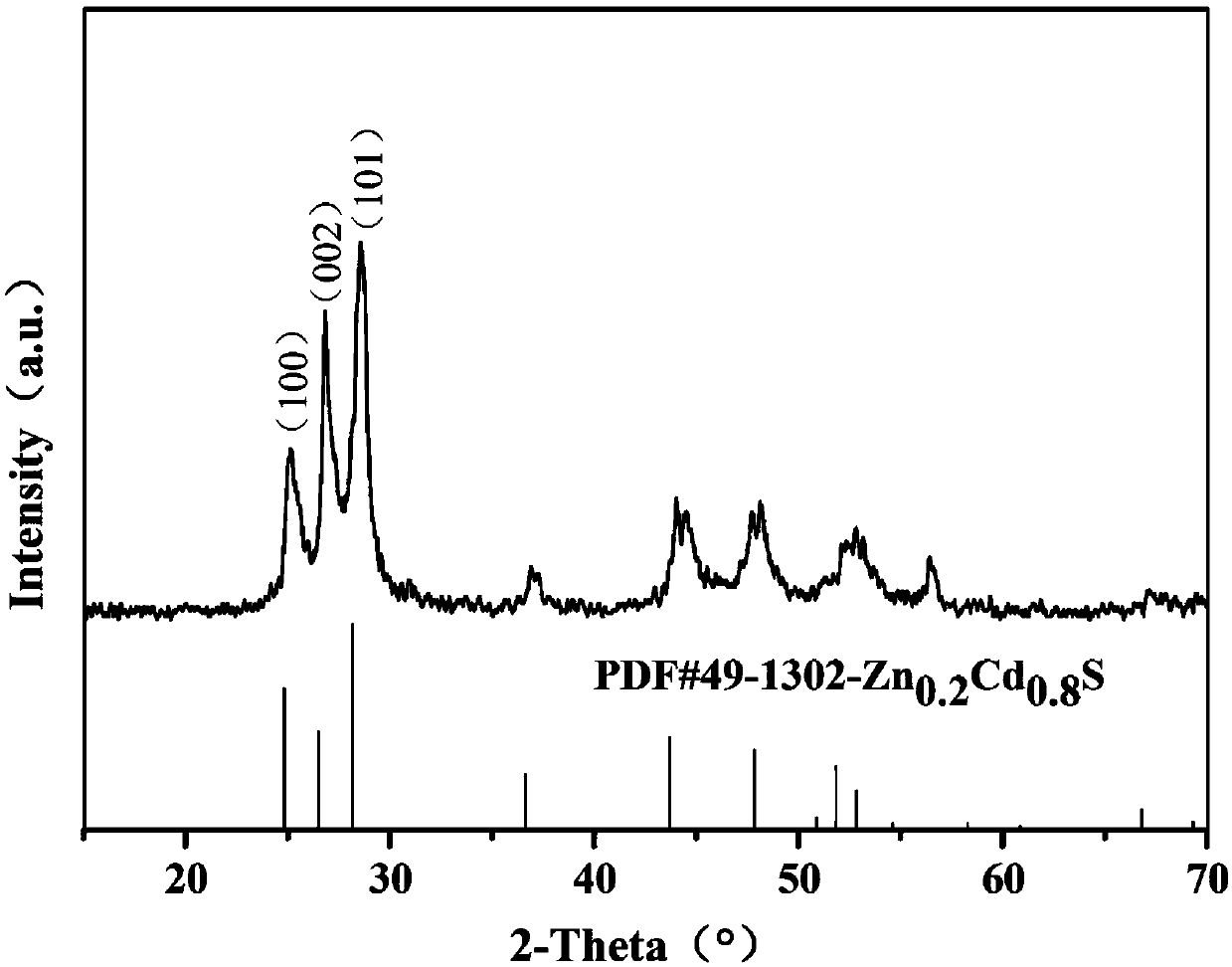
Preparation method of particulate self-assembling spherical zinc-cadmium-sulfur solid solution material
A self-assembly and solid solution technology, applied in chemical instruments and methods, cadmium compounds, inorganic chemistry, etc., can solve the problems of high energy and cost requirements for production, easy sintering or melting of products, and high temperature required for reactions. To achieve the effect of shortening the distance of electron transmission, benefiting environmental purification, good economic and social benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] A particle self-assembled spherical Zn 0.2 Cd 0.8 The preparation method of S material, comprises the following steps:
[0031] 1) Weigh a certain amount of CMC, make a solution (36ml) of 1-3g / L, perform magnetic stirring for 1-3 hours, and then perform ultrasonication for 5-30 minutes to form a mixed solution A.
[0032] 2) Using zinc acetate dihydrate (Zn(Ac) 2 2H 2O) and cadmium acetate dihydrate (Zn(Cd) 2 2H 2 O) is raw material (n Zn :n Cd =1:1), respectively weighed 0.1-0.5 mmol, added to mixed solution A, magnetically stirred for 15 minutes, and ultrasonicated for 5-30 minutes to form mixed solution B.
[0033] 3) Using thioacetamide as the sulfur source, weigh 1-3 mmol, add it into the mixed solution B, perform magnetic stirring for 15 minutes, and perform ultrasonication for 5-30 minutes to form the mixed solution C.
[0034] 4) Use the prepared HCl solution with a concentration of 0.01-0.1mol / L to acidify the mixed solution C, and use a pH tester to te...
Embodiment 1
[0039] 1) Weigh a certain amount of CMC, make it into a 1g / L solution (36ml), perform magnetic stirring for 1 hour, and then perform ultrasonication for 5 minutes to form solution A.
[0040] 2) Using zinc acetate dihydrate (Zn(Ac) 2 2H 2 O) and cadmium acetate dihydrate (Zn(Cd) 2 2H 2 O) is raw material (n Zn :n Cd =1:1), respectively weighed 0.1 mmol, added to solution A, magnetically stirred for 15 minutes, and ultrasonicated for 5 minutes to form mixed solution B.
[0041] 3) Using thioacetamide as the sulfur source, weigh 1 mmol, add it into the mixed solution B, perform magnetic stirring for 15 minutes, and perform ultrasonication for 5 minutes to form solution C.
[0042] 4) Use the prepared HCl solution with a concentration of 0.01mol / L to acidify solution C, and use a pH tester to test it. After the solution pH of the entire system is adjusted to PH=2.0, perform ultrasonication for 5 minutes to form a mixed solution d.
[0043] 5) The solution D is added to the...
Embodiment 2
[0046] 1) Weigh a certain amount of CMC, make it into a 2g / L solution (36ml), perform magnetic stirring for 2 hours, and then perform ultrasonication for 15 minutes to form solution A.
[0047] 2) Using zinc acetate dihydrate (Zn(Ac) 2 2H 2 O) and cadmium acetate dihydrate (Zn(Cd) 2 2H 2 O) is raw material (n Zn :n Cd =1:1), respectively weighed 0.3 mmol, added to solution A, magnetically stirred for 15 minutes, and ultrasonicated for 15 minutes to form mixed solution B.
[0048] 3) Using thioacetamide as the sulfur source, weigh 2 mmol, add it into the mixed solution B, perform magnetic stirring for 15 minutes, and perform ultrasonication for 15 minutes to form solution C.
[0049] 4) Use the prepared HCl solution with a concentration of 0.05mol / L to acidify the solution C, and use a pH tester to test it. After the pH of the entire system is adjusted to PH=2.5, perform ultrasonication for 10 minutes to form a mixed solution d.
[0050] 5) The solution D is added to the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com