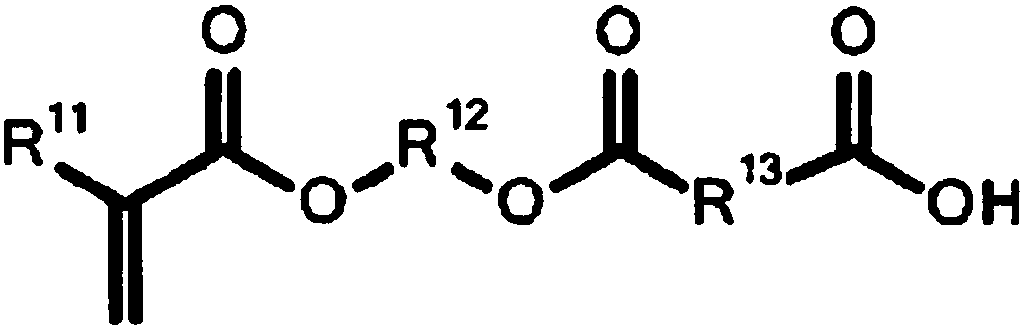
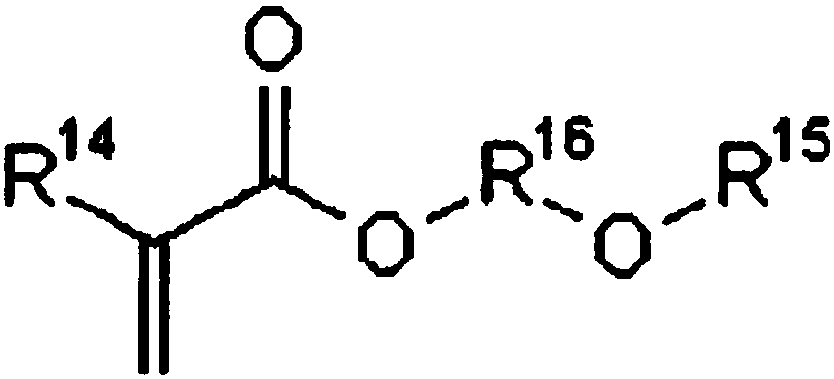
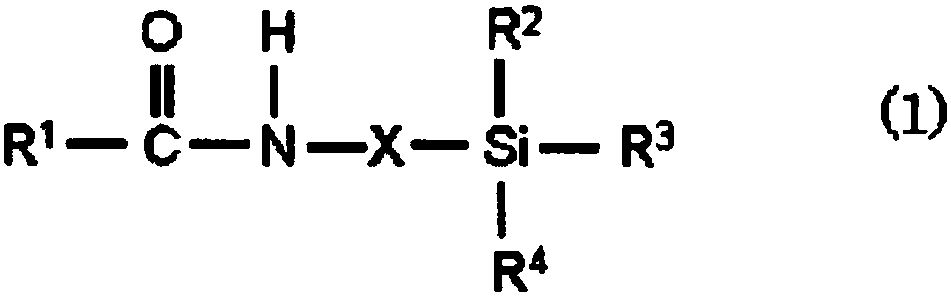Photosensitive composition, photosensitive composition for color filter, and color filter
A photosensitive composition, color filter technology, applied in the direction of filters, optics, optomechanical equipment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0589] Hereinafter, the embodiments of the present invention will be described more specifically through examples, but the following examples do not limit the scope of rights of the present invention in any way. In addition, "part" in an Example shows "mass part", and "%" shows "mass %".
[0590] In addition, in the following examples, the molecular weight of the resin is a polystyrene-equivalent weight average molecular weight measured by GPC (gel permeation chromatography), using GPC-8020 manufactured by Tosoh Corporation, using tetrahydrofuran as an eluent, Three TSKgelSuperHM-M columns (manufactured by Tosoh Corporation) were used for measurement at a flow rate of 0.6 ml / min, an injection volume of 10 μl, and a column temperature of 40°C.
[0591] The IR measurement was performed using an infrared spectrometer (Spectrum One) manufactured by PerkinElmer. The measurement of the acid value was performed as follows. About 1 g of the compound solution for acid value measureme...
manufacture example 1
[0597]Add 69.3 parts of propylene glycol monomethyl ether acetate, Desmodur (Desmodur) Z4470BA (isophorone di Isocyanate (Isophorone Diisocyanate, IPDI) isocyanurate, non-volatile content is 70% butyl acetate solution, isocyanate (Isocyanate, NCO) group content is 11.9%) 102.3 parts, furfuryl alcohol 28.4 parts, and dibutyltin dilaurate 0.20 parts were heated to 80° C. while injecting nitrogen gas into the container, stirring was continued for 8 hours, and IR measurement was performed to confirm that the target product was produced. After cooling to room temperature, it was diluted with propylene glycol monomethyl ether acetate to obtain a furyl group-containing compound solution A1 with a nonvolatile content of 20%. Furthermore, the compound does not contain an alkali-soluble group and does not contain a photopolymerizable functional group.
manufacture example 2
[0599] Add 61.3 parts of 2-methacryloyloxyethyl isocyanate, 38.7 parts of furfuryl alcohol, 0.10 parts of dibutyltin dilaurate, and 0.10 parts of methoxyphenol into a reaction vessel equipped with a stirrer, a thermometer, a reflux cooler, and a gas introduction pipe , while injecting dry air into the container, it was heated to 80° C., stirring was continued for 8 hours, and IR measurement was performed to confirm that the target product was produced. After cooling to room temperature, it took out without diluting, and obtained monomer 1 which is a furyl group-containing compound and furyl group-containing monomer (a-1). Furthermore, the compound does not contain an alkali-soluble group but contains a photopolymerizable functional group.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com