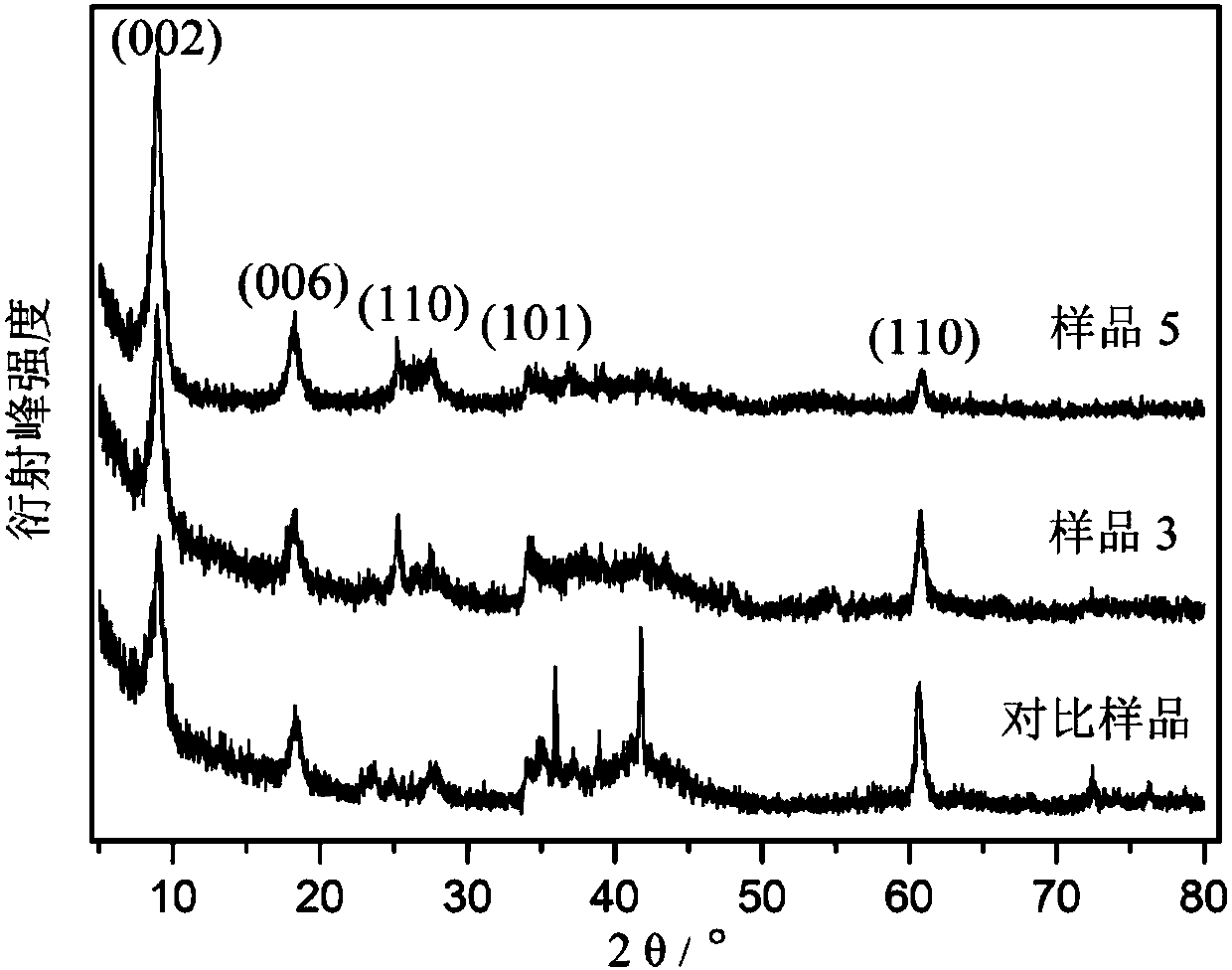
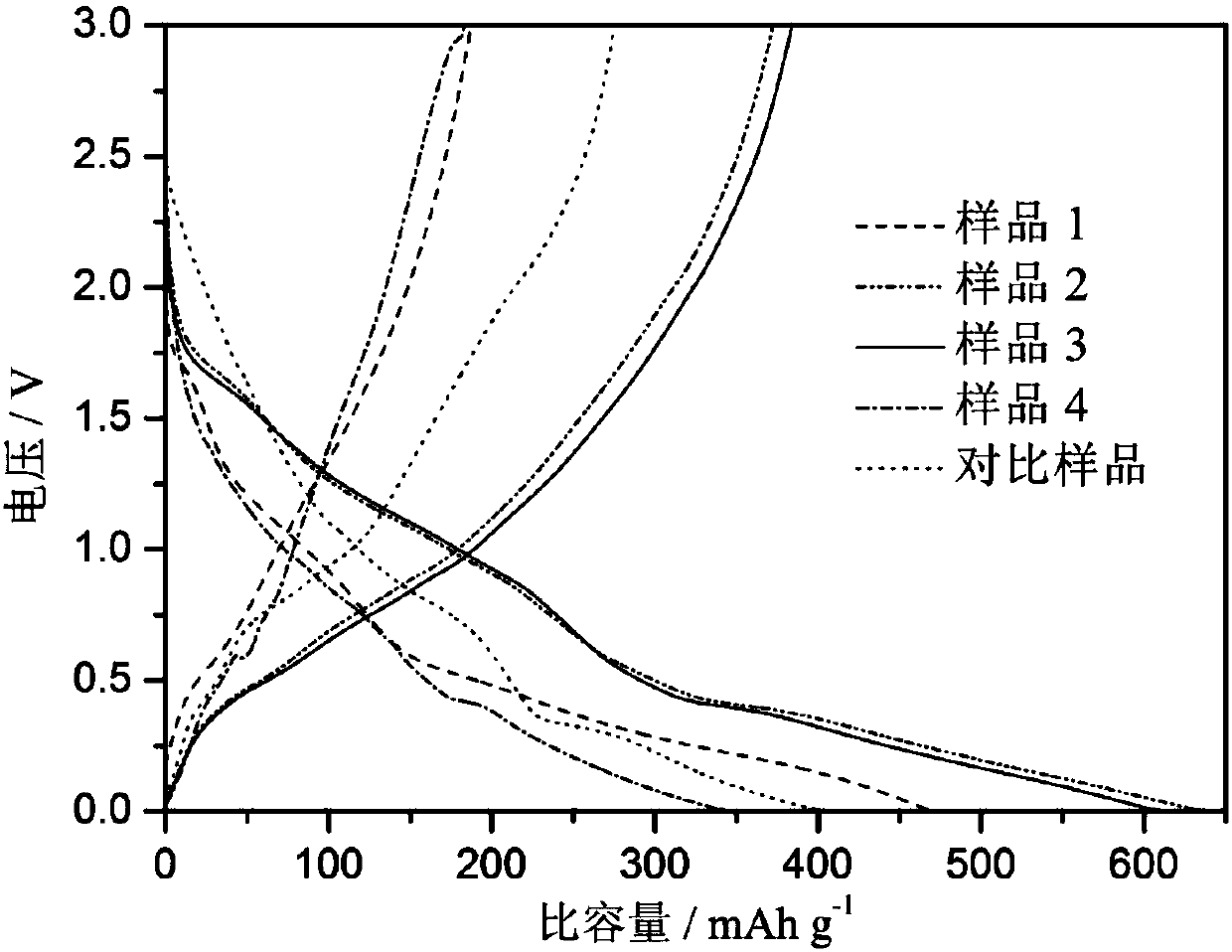
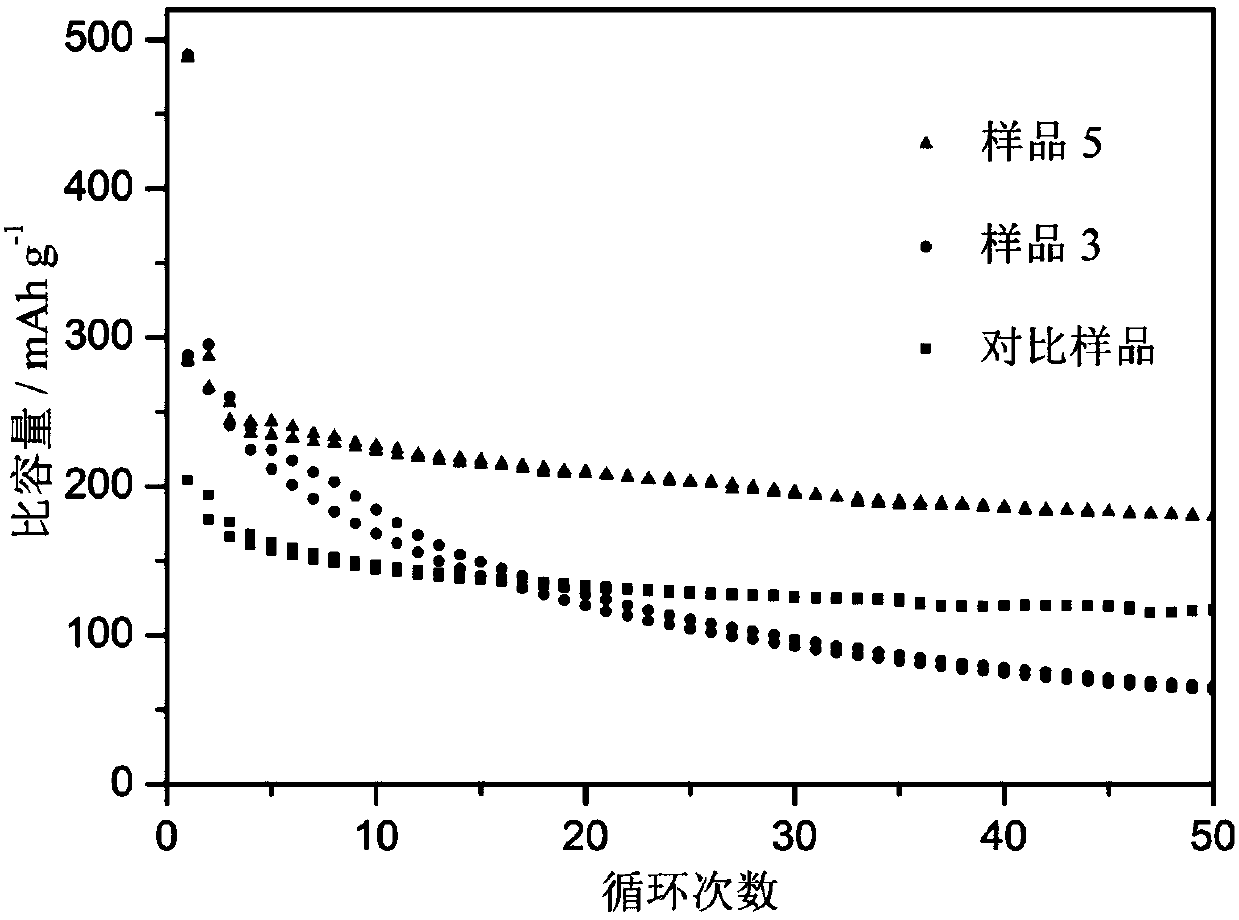
Carbon nanotube and tin dioxide modified titanium carbide lithium ion battery negative electrode material with three-dimensional 'plane-line-plane' structure and preparation method thereof
A technology of carbon nanotubes and tin dioxide, applied in battery electrodes, secondary batteries, structural parts, etc., to achieve the effect of improving electrochemical performance, excellent electrochemical performance, and improving surface-to-surface contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1 g Ti 3 AlC 2 The powder was dispersed into 20 ml of hydrofluoric acid solution with a mass fraction of 50%, and stood at room temperature for 24 hours, and then the precipitate was centrifuged with distilled water for 5 times until the pH of the solution was 5. The precipitate was put into a vacuum drying oven and dried at 80° C. for 12 hours to obtain a black powder. 0.113 g of SnCl 2 Dissolve in 25 mL of distilled water, then add the resulting black powder to SnCl 2 The solution was stirred at room temperature for 3 hours, and ultrasonic oscillation was continued for 30 minutes to obtain a black suspension. Put the black suspension into a reaction kettle with a polytetrafluoroethylene liner, then put the reaction kettle into an oven, and keep the temperature at 130°C for 5 hours. After natural cooling, wash the precipitate in the reaction kettle with distilled water three times, put the precipitate in a drying oven, and dry it at 80°C for 12 hours, then grind it...
Embodiment 2
[0043] 1 g Ti 3 AlC 2 The powder was dispersed into 20 ml of hydrofluoric acid solution with a mass fraction of 50%, and stood at room temperature for 24 hours, and then the precipitate was centrifuged with distilled water for 5 times until the pH of the solution was 5. The precipitate was put into a vacuum drying oven and dried at 80° C. for 12 hours to obtain a black powder. 0.564 g of SnCl 2 Dissolve in 25 mL of distilled water, then add the resulting black powder to SnCl 2 The solution was stirred at room temperature for 3 hours, and ultrasonic oscillation was continued for 30 minutes to obtain a black suspension. Put the black suspension into a reaction kettle with a polytetrafluoroethylene liner, then put the reaction kettle into an oven, and keep the temperature at 130°C for 5 hours. After natural cooling, wash the precipitate in the reaction kettle with distilled water three times, put the precipitate in a drying oven, and dry it at 80°C for 12 hours, then grind it...
Embodiment 3
[0046] 1 g Ti 3 AlC 2The powder was dispersed into 20 ml of hydrofluoric acid solution with a mass fraction of 50%, and stood at room temperature for 24 hours, and then the precipitate was centrifuged with distilled water for 5 times until the pH of the solution was 5. The precipitate was put into a vacuum drying oven and dried at 80° C. for 12 hours to obtain a black powder. 2.821 g of SnCl 2 Dissolve in 25 mL of distilled water, then add the resulting black powder to SnCl 2 The solution was stirred at room temperature for 3 hours, and ultrasonic oscillation was continued for 30 minutes to obtain a black suspension. Put the black suspension into a reaction kettle with a polytetrafluoroethylene liner, then put the reaction kettle into an oven, and keep the temperature at 130°C for 5 hours. After natural cooling, wash the precipitate in the reaction kettle with distilled water three times, put the precipitate in a drying oven, and dry it at 80°C for 12 hours, then grind it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com