A kind of racetam pharmaceutical composition containing buffer
A buffering agent and composition technology, applied in the field of medicine, can solve the problems of quality change, inability to apply clinically, non-conformity, etc., and achieve the effect of safe price, low price, and solving the effect of high temperature resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: Stability research test
[0038] Preparation of the racetam drug composition: the total mass volume percentage of the racetam drug and mannitol is within the range of 20% to 30%, placed at room temperature (25 o C), 4 o Under C conditions, observe the crystallization state after 10 days; at the same time, put another batch at -20 o C frozen for 2 days, then at 40 o C was placed for 2 days, and the reconstitution state was observed. The results are shown in Tables 2-4.
[0039] The racetam pharmaceutical composition and mannitol described in the present invention can be prepared by the following method, taking piracetam as an example.
[0040] According to Table 1, the piracetam and mannitol of the prescribed amount are dissolved in sterilized water for injection, and 0.5% ( w / v ) activated carbon, 100o C adsorption for 30 min, filtered while hot, potting, 121 o C sterilization for 8 min.
[0041]
[0042] The control group is a mannitol solutio...
Embodiment 2
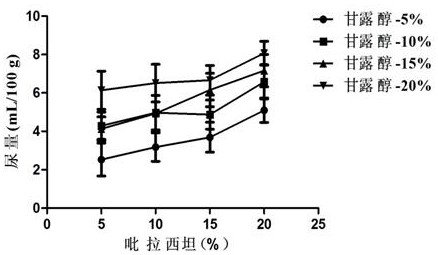
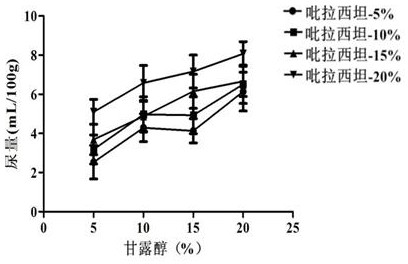
[0049] Embodiment 2: diuresis test
[0050] For acute diseases, such as acute cerebral edema induced by various etiologies, immediate dehydration is required. At this time, the combination of piracetam and mannitol is required to exert its high efficacy. The inventor selected several groups of compositions with different concentrations and carried out Diuretic test, the specific operation process is as follows:
[0051] Male Wistar rats, weighing 200 ± 20 g, were randomly divided into 18 groups, with 3 rats in each group, placed in metabolic cages for 1 day to adapt to the environment, and observed whether the urine output was stable under the condition of free drinking water. No food or water was allowed 18 h before the experiment. Following the diuretic screening method introduced by Aston, 2.2 mL / 100 g distilled water was administered into the stomach before the drug to maintain a certain water load in the animal body. Urine was collected for 2 hours. If the urine output ...
Embodiment 3
[0054] Embodiment 3: sodium acetate-dilute hydrochloric acid buffer solution
[0055] From Example 1, the mass volume ratio of piracetam to mannitol is 10:15 as an example, and the basic prescription is shown in Table 7.
[0056]
[0057] Sodium acetate-dilute hydrochloric acid buffer preparation method: Weigh 1.64 g sodium acetate into a 100 mL measuring bottle, add sterile injection solution to dissolve in water and dilute to the mark, adjust the pH to 3.00, 3.50, 4.00, 4.50 with dilute hydrochloric acid , 5.00, 5.50, 6.00, 6.50, 7.00 to make sodium acetate-dilute hydrochloric acid buffer solutions with different pH values.
[0058] Preparation method: Dissolve the piracetam and mannitol in the prescription amount according to Table 7 and dissolve them in sterile water for injection, add 0.5% ( w / v ) activated carbon, 100 o C adsorption for 30 min, filter while hot, add buffer, stir evenly, potting, 121 o C sterilized for 8 minutes, measured the pH value before and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com