Flocculation extraction separation method of zinc and cobalt ions
An extraction and flocculent technology, which is applied in the field of flocculent extraction and separation of zinc and cobalt ions, can solve the problems of poor extraction capacity, high production cost, and high price of P507, and achieve fewer extraction stages, low dosage, and good separation effect of zinc and cobalt Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
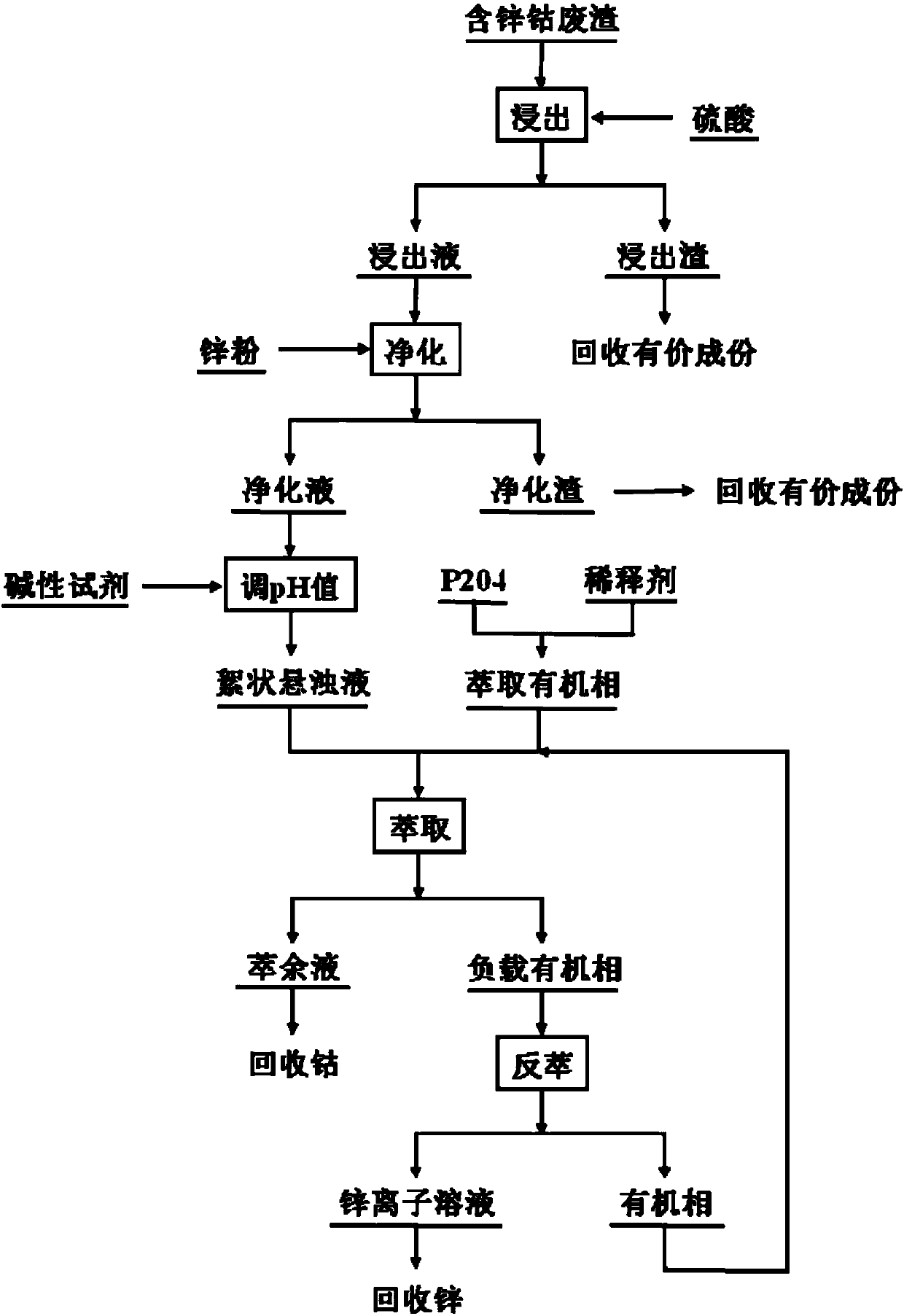
[0037]A method for flocculent extraction and separation of zinc-cobalt ions provided in this embodiment comprises the following steps:
[0038] (1) leaching waste residue containing zinc and cobalt with sulfuric acid, the excess coefficient of sulfuric acid is 1.2, the leaching time is 2.5h, the filtrate after leaching is leachate, and the leaching rate of zinc and cobalt reaches 99.7%;
[0039] (2) Add zinc powder to the leaching solution, the excess coefficient of zinc powder is 1.5, and the purification time is 0.8h to remove impurities such as copper and cadmium in the leaching solution. ;
[0040] (3) Add sodium hydroxide to the purification solution, adjust the pH value of the purification solution to 7.8, until the purification solution becomes a flocculent suspension;
[0041] (4) With P204 as the extractant, after adding sulfonated kerosene, mix and configure the extracted organic phase whose concentration of P204 is 0.3mol / L;
[0042] (5) Mix the flocculent suspens...
Embodiment 2
[0047] (1) leaching waste residue containing zinc and cobalt with sulfuric acid, the excess coefficient of sulfuric acid is 1.1, the leaching time is 2h, the filtrate after leaching is leachate, and the leaching rate of zinc and cobalt reaches 98.2%;
[0048] (2) Add zinc powder to the leaching solution, the excess coefficient of zinc powder is 1.2, the purification time is 0.9h, remove copper, cadmium and other impurity components in the leaching solution, the filtrate after impurity removal and filtration is the purification solution, and obtain purification residue ;
[0049] (3) Add sodium hydroxide to the purification solution, adjust the pH value of the purification solution to 7.5, until the purification solution becomes a flocculent suspension;
[0050] (4) Take P204 as the extraction agent, add sulfonated kerosene and mix to form an extraction organic phase whose concentration of P204 is 0.25mol / L;
[0051] (5) Mix the flocculent suspension and the extracted organic ...
Embodiment 3
[0056] (1) leaching waste residue containing zinc and cobalt with sulfuric acid, the excess coefficient of sulfuric acid is 1.3, the leaching time is 2h, the filtrate after leaching is leachate, and the leaching rate of zinc and cobalt reaches 99.6%;
[0057] (2) Add zinc powder in the leaching solution, the excess coefficient of the zinc powder is 1.3, and the purification time is 1h, remove the impurity components such as copper and cadmium in the leaching solution, the filtrate after the impurity removal and filtration is the purification solution, and obtain the purification slag;
[0058] (3) Add sodium hydroxide to the purification solution, adjust the pH value of the purification solution to 8.5, until the purification solution becomes a flocculent suspension;
[0059] (4) Take P204 as the extraction agent, add sulfonated kerosene and mix to form an extraction organic phase whose concentration of P204 is 0.1mol / L;
[0060] (5) Mix the flocculent suspension and the extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com