Preparation method of 2,4,6-trifluorobenzylamine
A technology of trifluorobenzylamine and trifluorobenzonitrile, which is applied in the field of preparation of 2,4,6-trifluorobenzylamine, can solve the problems of high corrosiveness of production equipment, unfavorable large-scale production, unfriendly environment, etc. Low production cost, short synthetic route steps, and environmentally friendly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
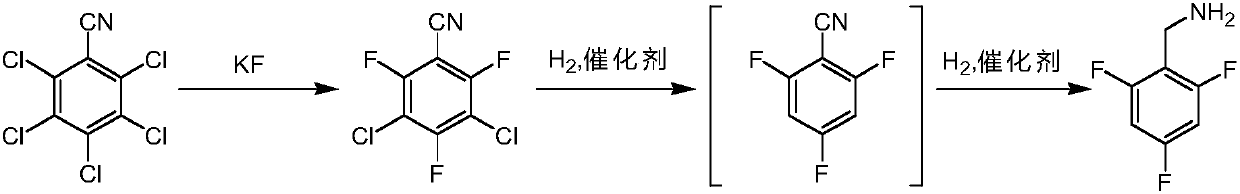
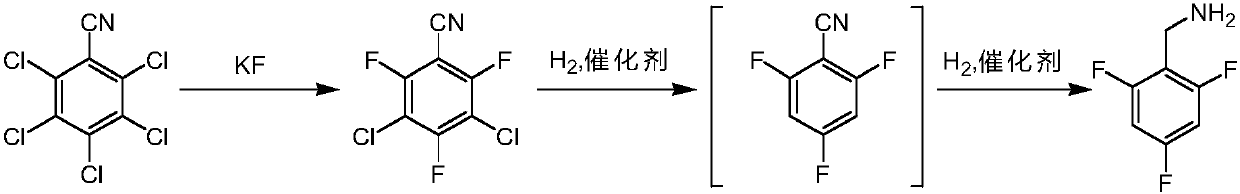
[0026] The invention provides a kind of preparation method of 2,4,6-trifluorobenzylamine, comprises the steps:
[0027] S1. Using pentachlorobenzonitrile as a starting material, perform fluorination reaction with anhydrous potassium fluoride in the first organic solvent to obtain 3,5-dichloro-2,4,6-trifluorobenzonitrile;
[0028] S2a. Add the 3,5-dichloro-2,4,6-trifluorobenzonitrile prepared in step S1 to the second organic solvent, add an organic base, and pass in hydrogen, under the action of the first catalyst, The intermediate 2,4,6-trifluorobenzonitrile was obtained through dechlorination and hydrogenolysis;
[0029] S3. Add the 2,4,6-trifluorobenzonitrile prepared in step S2a to the third organic solvent, add acid, and pass in hydrogen. Under the action of the second catalyst, the intermediate 2,4,6 -Trifluorobenzonitrile is reduced by cyano group to obtain 2,4,6-trifluorobenzylamine.
[0030] In the above technical scheme, after the fluorination reaction is finished, ...
Embodiment 1
[0046]Pentachlorobenzonitrile (20g, 72.6mmol) and anhydrous potassium fluoride (13.9g, 239.2mmol) were put into sulfolane (100mL), heated to 130-140°C under nitrogen protection, and kept for 3h. After the reaction was completed, the reaction liquid was lowered to room temperature, 100 mL of methyl tert-butyl ether and 200 mL of water were added, and the layers were extracted and separated. The organic phase was separated, washed with 100 mL of water, dried over anhydrous sodium sulfate, filtered, and precipitated under reduced pressure to obtain 3,5-dichloro-2,4,6-trifluorobenzonitrile (16.2 g, yield 98.6%), HPLC content 98.9%.
Embodiment 2
[0048] Pentachlorobenzonitrile (20g, 72.6mmol) and anhydrous potassium fluoride (13.9g, 239.2mmol) were put into sulfolane (100mL), heated to 130-140°C under nitrogen protection, and kept for 7h. After the reaction was completed, the reaction liquid was lowered to room temperature, 100 mL of methyl tert-butyl ether and 200 mL of water were added, and the layers were extracted and separated. The organic phase was separated, washed with 100 mL of water, dried over anhydrous sodium sulfate, filtered, and precipitated under reduced pressure to obtain 3,5-dichloro-2,4,6-trifluorobenzonitrile (13.8 g, yield 84.1%), HPLC content 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com