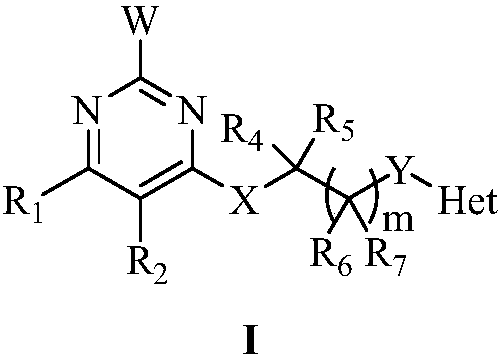
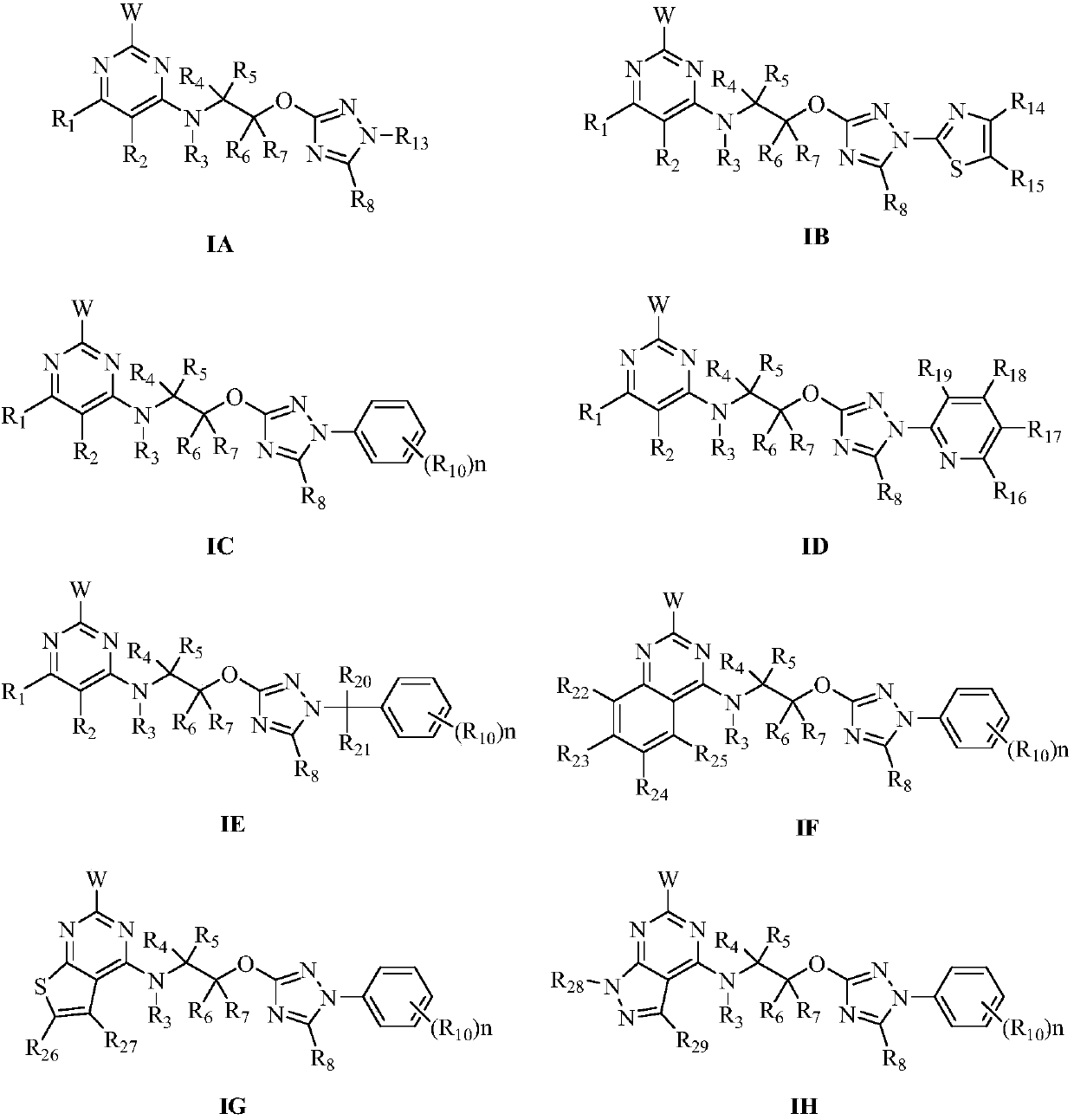
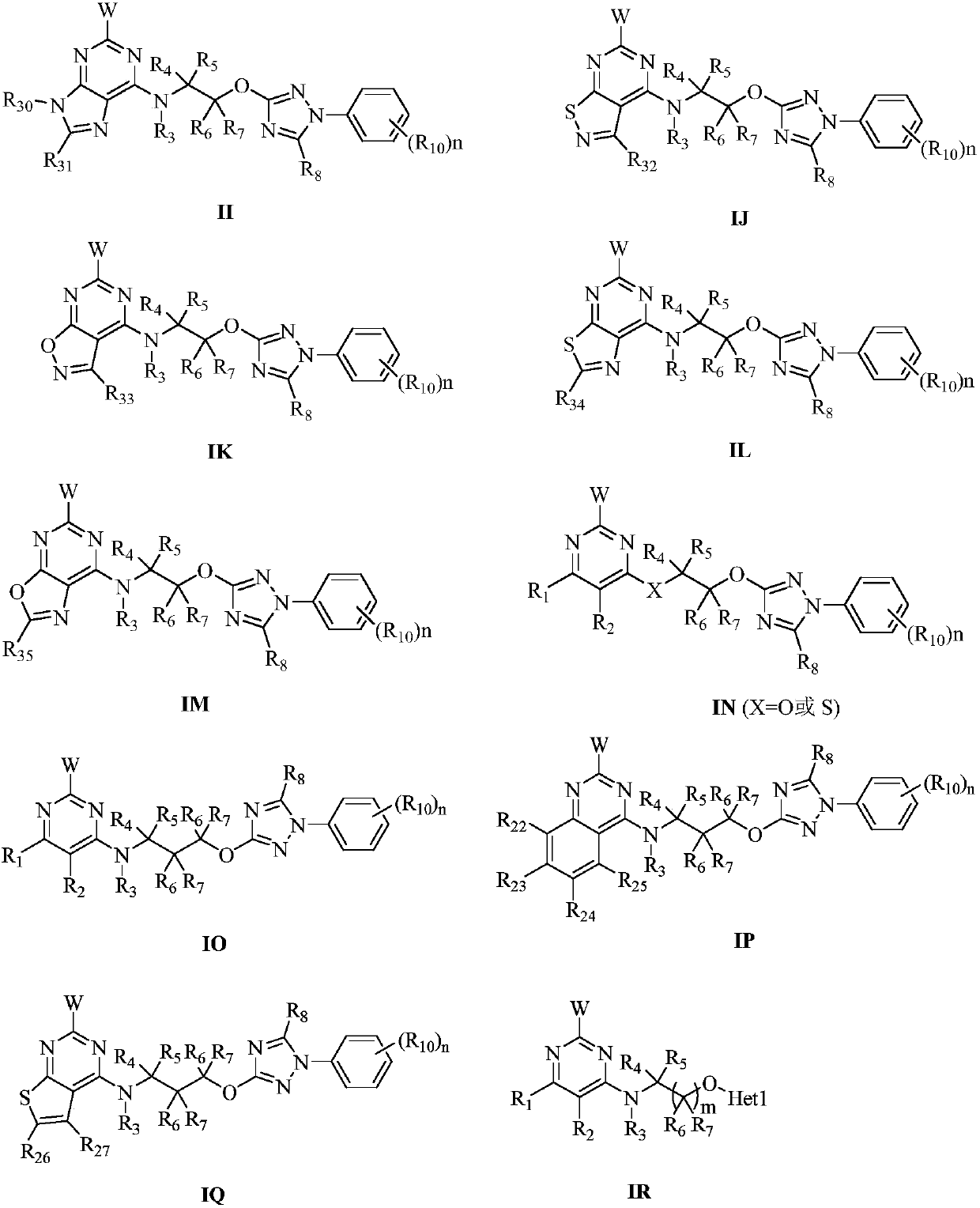
Pyrimidine-containing substituted azole compounds and application thereof
A technology of azole compounds and compounds, applied in the fields of chemicals, applications, and organic chemistry for biological control, which can solve problems such as unreported structural compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0400] Embodiment 1: the preparation of intermediate 4,5-dichloro-6-methylpyrimidine
[0401] 1) Preparation of 4-hydroxyl-5-chloro-6-methylpyrimidine
[0402]
[0403] Slowly add 8.80 g (0.16 mol) of sodium methoxide in methanol solution dropwise to 11.30 g (0.11 mol) of formamidine acetate in 50 ml of methanol under stirring at room temperature, and continue stirring at room temperature for 2 h after dropping. Then, 11.17 g (0.068 mol) of intermediate ethyl 2-chloroacetoacetate was added dropwise to the above solution, and the stirring reaction at room temperature was continued for 5-7 hours. After the completion of the reaction monitored by TLC, the solvent was evaporated under reduced pressure, and the pH was adjusted to 5-6 with hydrochloric acid, and an orange-yellow solid was obtained by suction filtration. The aqueous phase was extracted with (3×50ml) ethyl acetate, dried over anhydrous magnesium sulfate, filtered, and dissolve. The residue was dissolved in 50ml o...
Embodiment 2
[0407] Example 2: Preparation of 4,5-dichlorothieno[2,3-d]pyrimidine
[0408]
[0409] Get 2-amino-3-cyano-4-oxo-5,5-dihydrothiophene and 250ml phosphorus oxychloride (POCl 3 ) into the reaction bottle, slowly add 38ml of N,N-dimethylformamide dropwise at room temperature, and the dropwise addition is completed in about 30 minutes. React at room temperature for 1 hour, then raise the temperature to 75°C for 3 hours. After cooling down to room temperature, the reaction solution was poured into crushed ice and filtered to obtain 89.1 g of a dark gray solid with a yield of 86.9% and a melting point of 160-161°C.
Embodiment 3
[0410] Embodiment 3: the preparation of intermediate 4-chloroquinazoline
[0411] 1) Preparation of quinazolin-4(3H)-one
[0412]
[0413] Take 13.7g (0.1mol) of anthranilic acid and 20ml of formamide in a 250ml three-necked flask, and heat up to 140°C for 5-8 hours. After the completion of the reaction as monitored by TLC, the temperature of the reaction solution was lowered to 100° C., 80 ml of water was added dropwise with stirring, and then cooled to room temperature, filtered and washed with anhydrous ether to obtain 10.96 g of reddish brown, with a yield of 75.1%.
[0414] 2) Preparation of 4-chloroquinazoline
[0415]
[0416] Take 14.6g (0.1mol) of quinazolin-4(3H)-one in a 250ml single-necked bottle, use 50ml of thionyl chloride as a solvent, and raise the temperature to reflux for 4-6 hours. After the reaction was monitored by TLC, after cooling, the reaction solution was poured into water and stirred for 30 min, filtered and washed with anhydrous ether to ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com