Long-chain alkane polyurethane and preparation method and application thereof
A long-chain alkane, polyurethane technology, applied in the field of polyurethane materials, can solve the problems of limiting the application of polyurethane materials, molecular chain breakage, lack of new polyurethane materials, etc., and achieve the effect of excellent hydrolytic stability and hydrophobicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] As previously mentioned, the present invention provides a kind of preparation method of long-chain alkane polyurethane, comprising:
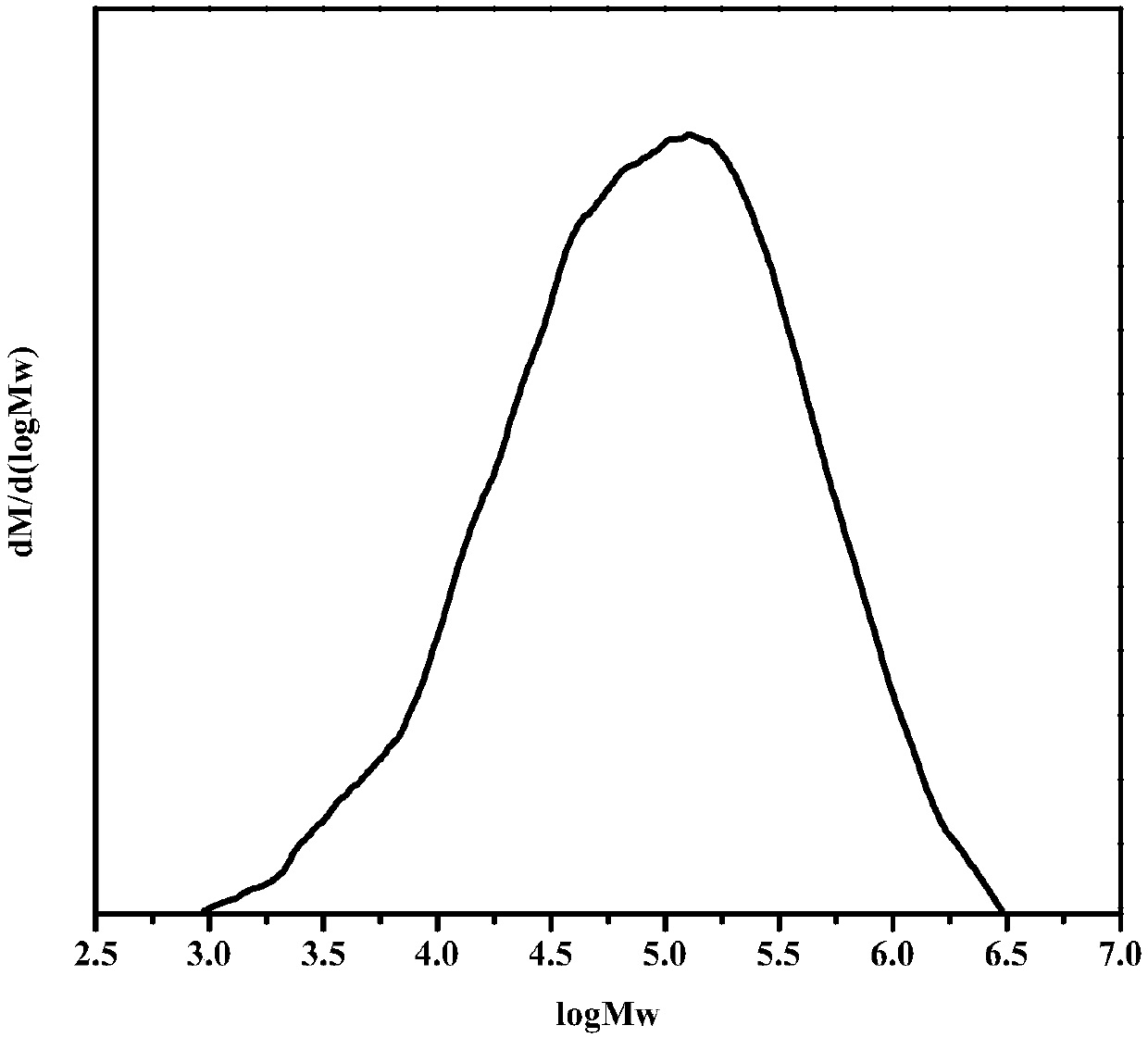
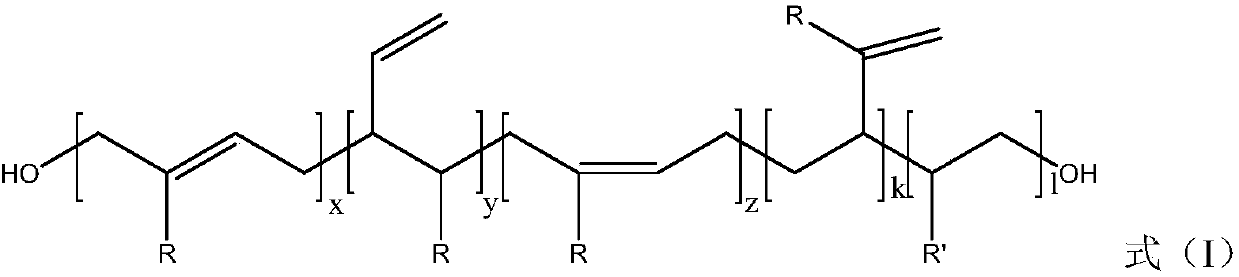
[0058] Using unsaturated diols and diisocyanates as raw materials to carry out addition and chain extension reactions to synthesize polyolefin polyurethanes; then carry out hydrogenation reactions on them to obtain the long-chain alkane polyurethanes; wherein, the unsaturated dihydric Alcohols include hydroxyl-terminated polyolefin diols. Wherein, the degree of hydrogenation of the carbon-carbon double bond in the olefin in the unsaturated dihydric alcohol is greater than or equal to 99 mol%; preferably, greater than or equal to 99.5 mol%.
[0059] In a preferred embodiment of the present invention, the preparation method of described long-chain alkane polyurethane specifically comprises:
[0060] (a) Take a certain amount of hydroxyl-terminated polyolefin diol and catalyst and add them to the solvent for thorough mixing. After fully mix...
Embodiment 1
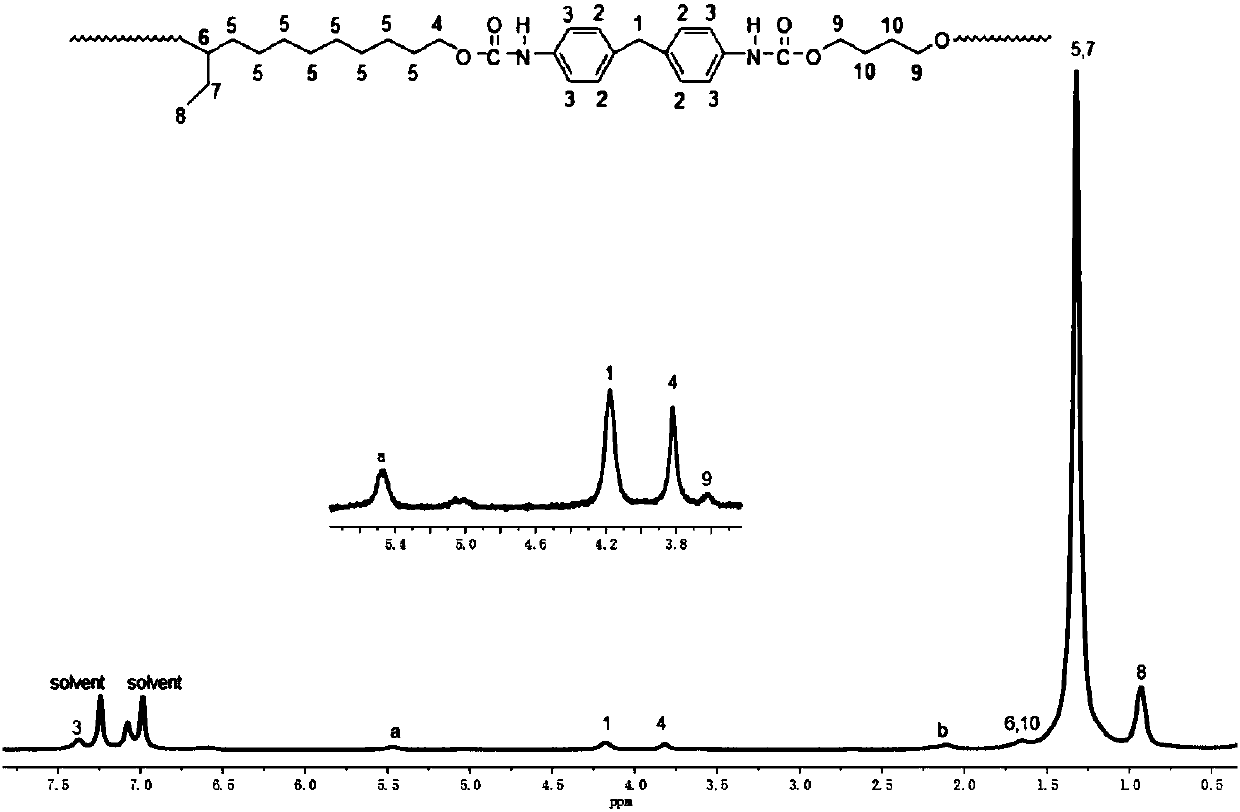
[0114] With double-terminated hydroxyl polybutadiene (R = 1.7, M n(HTPB) =3000g / mol) and diphenylmethane diisocyanate (MDI) are the synthesis of the long-chain alkane polyurethane of raw material
[0115] 5g dehydrated double-terminated polybutadiene (R=1.7, M n(HTPB) =3000g / mol) into a flask with a stirring magnet and a condenser tube, add 40mL of anhydrous toluene, 2 drops of dibutyltin dilaurate and 0.4mLN, N-dimethylformamide, set R=1.7 (isocyanate 0.7083g diphenylmethane diisocyanate corresponding to the ratio of alcoholic hydroxyl group to alcoholic hydroxyl group) was added to the constant pressure funnel, and 40mL of anhydrous toluene was added, microwave or oil bath was heated to 70°C, and diphenylmethane was added dropwise within 3h Toluene solution of diisocyanate, then continue to react at 70°C for 6h, then add 0.15g of chain extender 1,4-butanediol (BDO), and continue to react at this temperature for 5h, the above operations are all under the protection of nitrog...
Embodiment 2
[0119] With double-terminated hydroxyl polybutadiene-acrylonitrile copolymer (R = 1.8, M n =2000g / mol) and diphenylmethane diisocyanate (MDI) are the synthesis of the long-chain alkane polyurethane of raw material
[0120] 5g double-terminated hydroxyl polybutadiene-acrylonitrile copolymer (R=1.8, M n =2000g / mol) into a flask with a stirring magnet and a condenser tube, add 40mL of anhydrous toluene, 2 drops of dibutyltin dilaurate and 0.4mLN, N-dimethylformamide, make R=1.8 (isocyanate 1.126g of diphenylmethane diisocyanate corresponding to the ratio of alcoholic hydroxyl group to alcoholic hydroxyl group) was added to the constant pressure funnel, and 40mL of anhydrous toluene was added, microwave or oil bath was heated to 70°C, and the diphenylmethyl diisocyanate was added dropwise within 3h The diisocyanate toluene solution was reacted at 70°C for 3 hours, and then 0.25 g of chain extender 1,4-butanediol (BDO) was added, and the reaction was continued for 5 hours at this ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com