Catalyst for catalyzing copolymerization reaction of carbon dioxide and cyclohexene oxide to prepare poly(cyclohexene carbonate)
A technology of polycyclohexenyl carbonate and epoxycyclohexane, which is applied in the field of organic synthesis, can solve the problems of complex preparation process and high catalyst cost, and achieve the effects of good reaction selectivity, simple preparation process and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Catalyst 2, the preparation method of 5-bis (2-formylphenoxymethyl) furan condensed 3-aminothiophene zinc complex is as follows:
[0025] (1) Dissolve 0.99g (0.01mol) of 3-aminothiophene and 1.68g (0.005mol) of 2,5-bis(2-formylphenoxymethyl)furan in 20mL of methanol respectively to form a solution. 2,5-bis(2-formylphenoxymethyl)furan methanol solution was added dropwise to 3-aminothiophene methanol solution, heated to reflux after the dropwise addition, stirred for 0.5h, washed with ether, dried to obtain 2,5- Bis(2-formylphenoxymethyl)furyl 3-aminothiophene ligand;
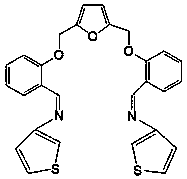
[0026] The structural formula of 2,5-bis(2-formylphenoxymethyl)furyl condensed 3-aminothiophene ligand is
[0027]
[0028] Structural characterization of ligands:
[0029] 1 H NMR (400 MHz, CDCl 3 ): δ 5.3(s, 4H, CH 2 ), 6.1~8.0(m, 16H, aromatic H), 8.4 (s, 2H, CH);
[0030] (2) Weigh 0.1 mol of 2,5-bis(2-formylphenoxymethyl)furyl 3-aminothiophene ligand into a container, vacuumize, fill with nit...
Embodiment 2
[0032] Catalyst 2, the preparation method of 5-bis (2-formylphenoxymethyl) furan condensed 3-aminothiophene zinc complex is as follows:
[0033] (1) Dissolve 0.99g (0.01mol) of 3-aminothiophene and 1.35g (0.004mol) of 2,5-bis(2-formylphenoxymethyl)furan in 15mL of methanol respectively to form a solution. 2,5-bis(2-formylphenoxymethyl)furan methanol solution was added dropwise to 3-aminothiophene methanol solution, heated to reflux after the dropwise addition, stirred for 1 h, washed with ether, dried to obtain 2,5-di (2-Formylphenoxymethyl)furyl 3-aminothiophene ligand.
[0034] (2) Weigh 0.1 mol of 2,5-bis(2-formylphenoxymethyl)furyl 3-aminothiophene ligand into a container, vacuumize, fill with nitrogen, and add 200mL of toluene to dissolve 2 , 5-bis(2-formylphenoxymethyl)furyl 3-aminothiophene ligand, add toluene solution (10%vol) containing 0.12mol diethylzinc under ice-salt bath cooling, and stir for 0.8h , remove the ice-salt bath, stir at room temperature for 1.3h, a...
Embodiment 3
[0036] Catalyst 2, the preparation method of 5-bis (2-formylphenoxymethyl) furan condensed 3-aminothiophene zinc complex is as follows:
[0037] (1) Dissolve 0.99g (0.01mol) of 3-aminothiophene and 2.02g (0.006mol) of 2,5-bis(2-formylphenoxymethyl)furan in 15mL of methanol respectively to form a solution. 2,5-bis(2-formylphenoxymethyl)furan methanol solution was added dropwise to 3-aminothiophene methanol solution, heated to reflux after the dropwise addition, stirred for 2 hours, washed with ether, dried to obtain 2,5-di (2-Formylphenoxymethyl)furyl 3-aminothiophene ligand.
[0038] (2) Weigh 0.1 mol of 2,5-bis(2-formylphenoxymethyl)furyl 3-aminothiophene ligand into a container, vacuumize, fill with nitrogen, and add 200mL of toluene to dissolve 2 , 5-bis(2-formylphenoxymethyl)furyl 3-aminothiophene ligand, add toluene solution (10%vol) containing 0.13mol diethylzinc under ice-salt bath cooling, after stirring for 1h, Remove the ice-salt bath, stir at room temperature for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com