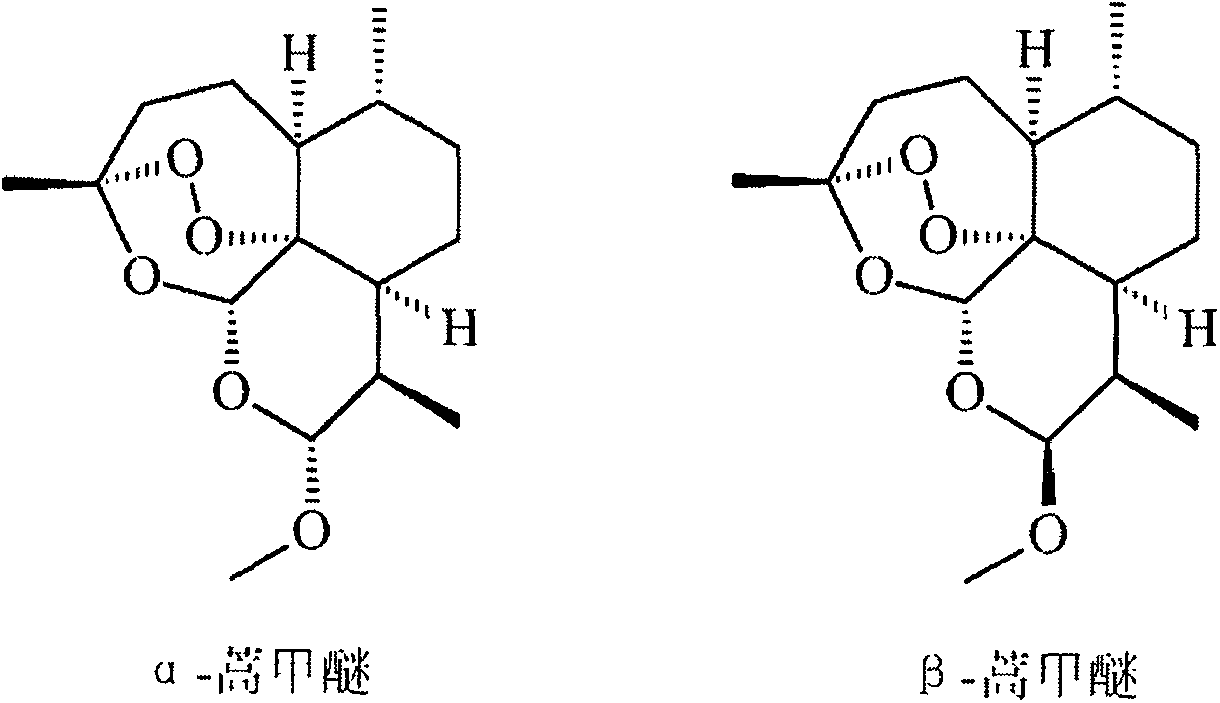
Preparation method of artemether
A technique for artemether and methanol, which is applied in the field of medicinal chemical synthesis, can solve the problems of complicated operation, low yield and low β-artemether content, and achieves the effects of reduced production cost, short technological process and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The invention discloses a preparation method of β-artemether. Those skilled in the art can refer to the content of this article and appropriately improve the process parameters to realize it. It needs to be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention, and relevant personnel can obviously make changes without departing from the content, spirit and scope of the present invention. Changes or appropriate changes and combinations are made to the content described herein to realize and apply the technology of the present invention.
[0019] In the present invention, unless otherwise specified, the scientific and technical terms used herein have the meanings commonly understood by those skilled in the art.
Embodiment 1
[0022] Dissolve 100g (0.35mol) of artemisinin in 1L of methanol, cool down to -2°C, slowly add 40g (1.1mol) of sodium borohydride under stirring, monitor the progress of the reaction by TLC, wait for the reaction to end, naturally rise to room temperature, drop Add saturated ammonium chloride solution to neutralize until neutral, extract three times with 200mL dichloromethane, wash with water, and dry over anhydrous sodium sulfate;
[0023] Cool the above organic solution to -2°C, add 22g (0.21mol) trimethyl orthoformate, stir for 30min, add dropwise 35mL (about 0.07mol) of 30% boron trifluoride ether solution in ether, after the dropwise addition is complete, continue the reaction 1 hour, naturally rise to room temperature, monitor the reaction progress, after the reaction is completed, slowly add saturated sodium bicarbonate solution dropwise, adjust the system to neutral, separate the liquid, extract the aqueous phase twice with 50mL dichloromethane, combine the organic phas...
Embodiment 2
[0026] Dissolve 100g (0.35mol) of artemisinin in 1L of methanol, cool down to -2°C, slowly add 45g (0.83mol) of potassium borohydride under stirring, monitor the progress of the reaction by TLC, wait for the reaction to end, naturally rise to room temperature, drop Add saturated ammonium chloride solution to neutralize until neutral, extract three times with 200mL dichloromethane, wash with water, and dry over anhydrous sodium sulfate;
[0027] Cool the above organic solution to -2°C, add 21g (0.20mol) of trimethyl orthoformate, stir for 30min, add dropwise 35mL (about 0.07mol) of 30% boron trifluoride ether solution in ether, after the dropwise addition is complete, continue the reaction 1 hour, naturally rise to room temperature, monitor the reaction progress, after the reaction is completed, slowly add saturated sodium bicarbonate solution dropwise, adjust the system to neutral, separate the liquid, extract the aqueous phase twice with 50mL dichloromethane, combine the organ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com