Long side chain type fluorine-containing sulfonated polyarylether compound based on bisphenol S and preparation method of long side chain type fluorine-containing sulfonated polyarylether compound
A technology of sulfonated polyarylethers and compounds, which is applied in the field of proton exchange membrane materials, can solve the problems of further improvement of proton conductivity, limited side chain length of sulfonated products, and harsh polymerization conditions, so as to improve oxidation stability and price Inexpensive, good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of fluorine-containing polyarylether compounds
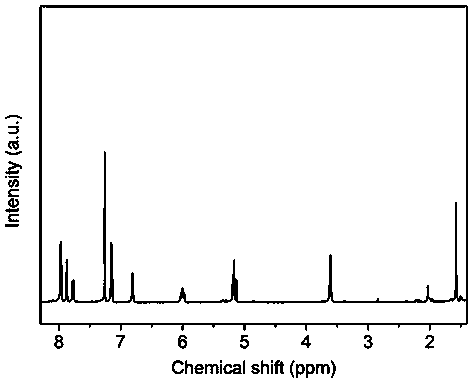
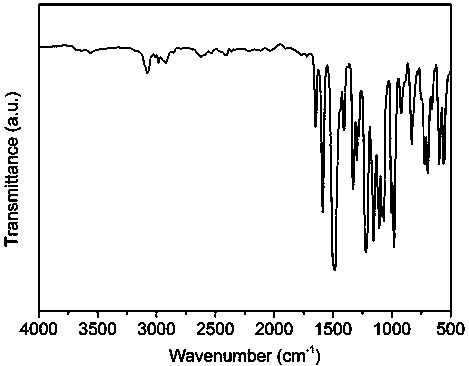
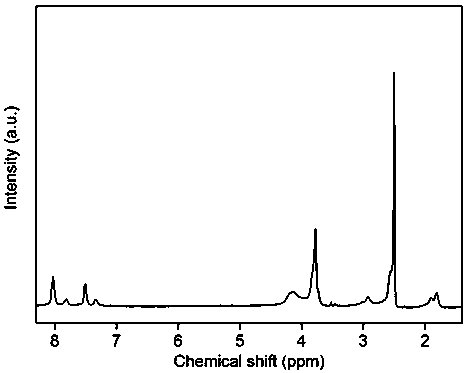
[0035] 1.0125 g (3.0 mmol) decafluorobiphenyl and 0.4204 g (1.5 mmol) bisphenol S, 0.4956 g (1.5 mmol) 2,2'-diallyl bisphenol S, 0.6836 g (4.5 mmol) cesium fluoride, Add 0.05 g of calcium hydride (for drying) and 7 mL of N-methylpyrrolidone into a three-necked flask, and magnetically stir at room temperature for 24 hours under the protection of argon. After the reaction, pour the reaction solution into aqueous methanol (1 : 1, v / v), filtered, collected and dried in a vacuum oven at 60-100°C for 10-40 hours to obtain a fluorinated polyarylether compound with an intrinsic viscosity of 1.2 dL / g and a yield of 95%. The data of the proton nuclear magnetic resonance spectrum of this compound are: 1 H NMR (400 MHz, CDCl 3 , ppm)δ 3.62 (d, 2H), 5.17 (t, 2H),5.99 (m, 1H), 6.80 (d, 1H), 7.15 (d, 1H), 7.75 (d, 2H), 7.87 (s, 1H), 7.98(d, 2H). The infrared data is: FT-IR (cm -1 ) υ 2929, 1648, 1591, 1484, 1...
Embodiment 2
[0036] Example 2 Preparation of Fluorine-Containing Polyarylether Compounds
[0037] Change the feeding amount of bisphenol S in Example 1 to 0.5256 g (2.1 mmol), and the feeding amount of 2,2'-diallyl bisphenol S to 0.2974 g (0.9 mmol), and the rest of the operations are as in Example 1 , the intrinsic viscosity of the obtained fluorine-containing polyarylether compound was 0.9dL / g, and the yield was 93%.
Embodiment 3
[0038] Example 3 Preparation of Fluorine-Containing Polyarylether Compounds
[0039] Change the feeding amount of bisphenol S in Example 1 to 0.2252 g (0.9 mmol), and the feeding amount of 2,2'-diallyl bisphenol S to 0.6938 g (2.1 mmol), and the rest of the operations are as in Example 1 , the intrinsic viscosity of the obtained fluorine-containing polyarylether compound was 1.1 dL / g, and the yield was 91%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com