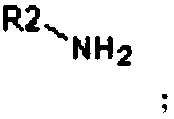Method for preparing organic aromatic amine compound
An amide compound and compound technology, applied in the field of preparation of organic aromatic amine compounds, can solve the problems of poor compatibility, many side reactions, poor versatility and the like, and achieve the effects of high compatibility, short reaction time and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] A method for preparing an organic aromatic amine compound. Add an inert amide compound A and a primary amine compound B to a solvent C, add a catalyst D and an inorganic salt E, and heat to 110° C. for 20 hours in a protective gas atmosphere; post-reaction treatment Obtain an organic aromatic amine compound;
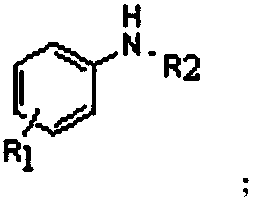
[0050] The structural formula of the organic aromatic amine compound is:
[0051]
[0052] The R1 group is -OCH; the R2 group is
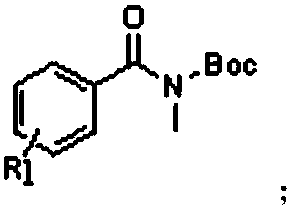
[0053] Specifically, the structural formula of the inert amide compound A is as follows:
[0054] The R1 group is -OCH 3 .
[0055] Specifically, the structural formula of the primary amine compound B is as follows:
[0056] The R2 group is
[0057] Specifically, the solvent C is mesitylene.
[0058] Specifically, the catalyst D is nickel acetate and a carbene ligand.
[0059] Specifically, the molar ratio of the nickel acetate to the carbene ligand is 1:2; the structural formula of the carbene ligand is as follows: ...
Embodiment 2
[0068] A method for preparing an organic aromatic amine compound. Add an inert amide compound A and a primary amine compound B to a solvent C, add a catalyst D and an inorganic salt E, and heat to 120° C. for 10 hours in a protective gas atmosphere; post-reaction treatment Obtain an organic aromatic amine compound;
[0069] The structural formula of the organic aromatic amine compound is:
[0070] The R1 group is -CH 3 ; The R2 group is
[0071] Specifically, the structural formula of the inert amide compound A is as follows:
[0072] The R1 group is -CH 3 .
[0073] Specifically, the structural formula of the primary amine compound B is as follows:
[0074] The R2 group is
[0075] Specifically, the solvent C is mesitylene.
[0076] Specifically, the catalyst D is nickel acetate and a carbene ligand.
[0077] Specifically, the molar ratio of the nickel acetate to the carbene ligand is 1:2; the structural formula of the carbene ligand is as follows:
[0078]...
Embodiment 3
[0086] A method for preparing an organic aromatic amine compound. Add an inert amide compound A and a primary amine compound B to a solvent C, add a catalyst D and an inorganic salt E, and heat to 115° C. for 15 hours in a protective gas atmosphere; post-reaction treatment Obtain an organic aromatic amine compound;
[0087] The structural formula of the organic aromatic amine compound is:
[0088] The R1 group is -C 2 h 5 ; The R2 group is
[0089] Specifically, the structural formula of the inert amide compound A is as follows:
[0090] The R1 group is -C 2 h 5 .
[0091] Specifically, the structural formula of the primary amine compound B is as follows:
[0092] The R2 group is
[0093] Specifically, the solvent C is mesitylene.
[0094] Specifically, the catalyst D is nickel acetate and a carbene ligand.
[0095] Specifically, the molar ratio of the nickel acetate to the carbene ligand is 1:2; the structural formula of the carbene ligand is as follows: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com