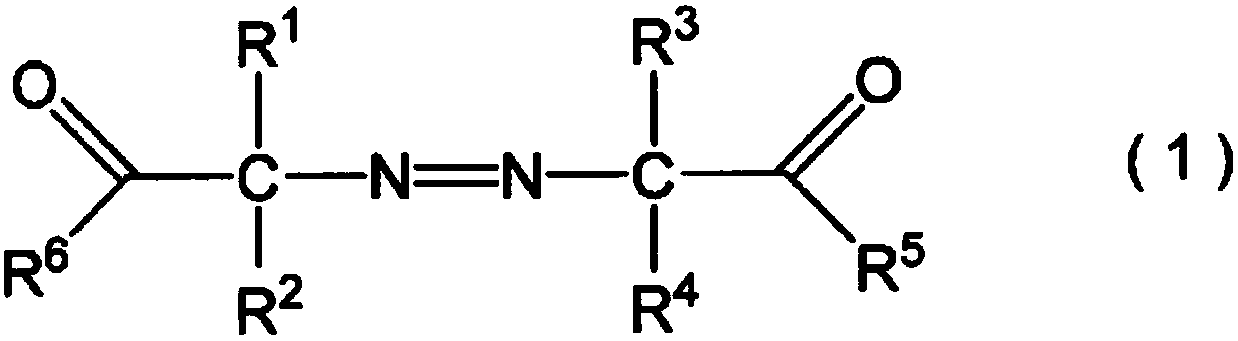
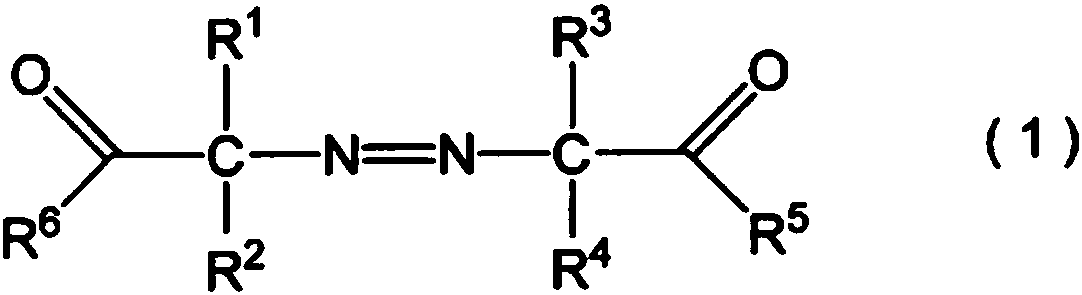
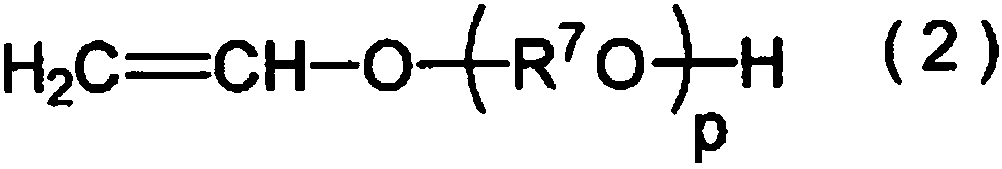Improved method for producing hydroxy group-containing vinyl ether polymer
A technology of vinyl ether and manufacturing method, applied in the direction of unsaturated ether copolymer adhesive, adhesive type, application, etc., can solve the problem of lower monomer conversion rate, inability to perform efficient polymerization, vinyl ether polymer Yield decline and other problems, to achieve the effect of inhibiting the formation of polyacetal and improving the conversion rate of monomer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1: the manufacture of poly(2-hydroxyethyl vinyl ether) (1)
[0095] A stirrer, and 2-hydroxyethyl vinyl ether (hereinafter referred to as "HEVE") as a monomer, 500 mole parts, MAIB (Wako Pure Chemical Industries, Ltd.) as a radical polymerization initiator were put into the test tube ) product, trade name V-601) 1 mole part (0.2 mole % with respect to the monomer), further adding water as a polymerization solvent so that the HEVE concentration becomes 50 mass %, and fully dissolved it. Next, nitrogen gas was blown into the test tube to remove oxygen. After removing the oxygen, the test tube was sealed, and the polymerization reaction was started in an oil bath at 70°C. After 48 hours, the polymerization was terminated by cooling and exposure to air, and 1 H NMR analysis and GPC analysis. As a result, the monomer conversion after 48 hours had passed was 99% or more, the number average molecular weight Mn of the obtained polymer was 33900, and the molecula...
Embodiment 2
[0096] Embodiment 2: the manufacture of poly(2-hydroxyethyl vinyl ether) (2)
[0097] The polymerization reaction was performed in the same manner as in Example 1 except that water was added so that the HEVE concentration became 20% by mass. As a result, the monomer conversion after 48 hours had passed was 99% or more, the number average molecular weight Mn of the obtained polymer was 10700, and the molecular weight distribution Mw / Mn was 1.59.
Embodiment 3
[0098] Embodiment 3: the manufacture of poly(2-hydroxyethyl vinyl ether) (3)
[0099] 2,2'-Azobis[2-methyl-N-(2-hydroxyethyl)propionamide] (manufactured by Wako Pure Chemical Industries, Ltd., trade name V-086) was used as a radical polymerization initiator , except that, the polymerization reaction was carried out by the same operation as in Example 1. As a result, the monomer conversion after 93 hours had passed was 99% or more, the number average molecular weight Mn of the obtained polymer was 11400, and the molecular weight distribution Mw / Mn was 1.94.
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com