Porcine rotavirus VP6 and VP7 combined vaccine
A vaccine adjuvant and subunit vaccine technology, applied in the field of vaccine preparation, can solve the problems of high porcine rotavirus infection rate and insufficient research and development of porcine rotavirus vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Screening and sequence analysis of VP6 gene
[0024] In recent years, the gene sequence analysis of porcine rotavirus (RV) shows that there are differences between the genome sequences of different strains, and there are obvious "drift" and "transformation" phenomena at the same time, resulting in the existence of multiple serotypes of RV. One reason for this phenomenon is that when two or more RV viruses invade a host cell at the same time, the gene segments interact, and exchange, crossover or rearrangement occur during transcription and replication, causing virus mutations. Thus, segmental rearrangements among different strains and mutations within the genome lead to the generation of mutant strains. Mutant strains have a certain probability of reducing the immune effect of existing vaccines or having no effect at all. The serum cross-neutralization test can be used to detect whether the existing vaccine antiserum can effectively neutralize the screened m...
Embodiment 2
[0079] Example 2: Expression of VP6 antigen
[0080] 1. Cloning of target gene and construction of recombinant expression plasmid
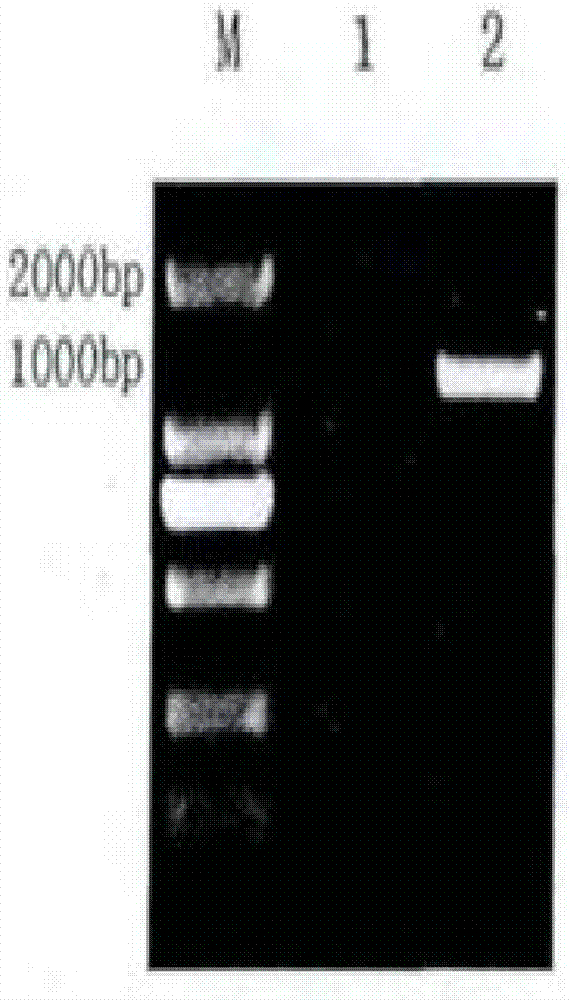
[0081] Add 10 μl of artificially synthesized plasmid DNA to E.coli Top10 and mix in ice bath for 30 minutes, then heat shock at 42°C for 90 seconds, and then immediately ice bath for 2‐3 minutes. Then spread evenly on LB plates containing ampicillin (100 μg / ml) and culture overnight at 37°C. Pick the positive colonies and shake the bacteria, extract the plasmid, and identify the correct recombinant plasmid by PCR and double enzyme digestion (NcoI / XhoI), and send it to Sangon Bioengineering (Shanghai) Co., Ltd. for sequencing. The recombinant cloning plasmid and expression vector pGEX-4T-1 were digested with NcoI and XhoI respectively, the gene fragment and the pGEX-4T-1 vector fragment were recovered, ligated overnight at 16°C with T4 DNAse, and the ligated product was transformed into Escherichia coli Top 10 In the process, positive colonies we...
Embodiment 3
[0084] Example 3: Neutralizing effect of VP6 antigen obtained antibody serum on virus strains
[0085] According to the method described in Example 2, the VP6 recombinant protein (amino acid sequence is SEQ ID NO: 3) numbered as Sequence ID: AFD33516.1 in NCBI is recombinantly expressed, and the amino acid sequence prepared in Example 2 is SEQ ID NO: 2. The VP6 antigen protein was used as an immune antigen to immunize mice to prepare antibody serum, and the serum was subjected to neutralization experiments to detect immune performance.
[0086] The purified recombinant protein was absolutely quantified by spectrophotometry according to the following steps:
[0087] (1) Add 0.15ml of PBS for protein measurement and 2.85ml of Coomassie Brilliant Blue staining solution for protein quantification in the cuvette as a control, and perform zero adjustment;
[0088] (2) Add 0.14ml of PBS for the protein to be tested, 0.01ml of the protein solution to be tested and 2.85ml of Coomassie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com