Method for synthesizing acyl naphthalene by using micro-channel reactor
A microchannel reactor and reactor technology, applied in the direction of condensation preparation of carbonyl compounds, separation/purification of carbonyl compounds, organic chemistry, etc., can solve the problems of long reaction time, low product yield, and no mention of product purification, etc. To achieve the effect of short material residence time, reduce reaction time, and increase reaction kinetic rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
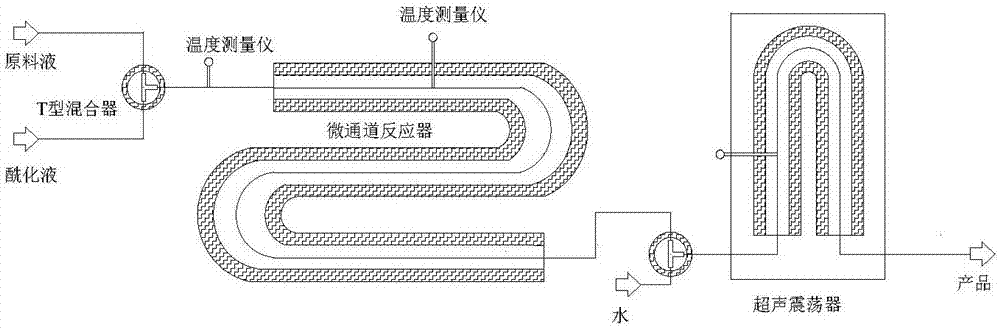
[0020] Put the stirred reactor into a low-temperature tank, add 15g of nitrobenzene, then add 2.5g of anhydrous aluminum trichloride, and finally add 1.3g of propionyl chloride, control the temperature at 0°C, and react for 10 minutes to prepare acylation liquid. Add 15g of nitrobenzene into a stirred reactor, and then add 1.5g of 2-methylnaphthalene to prepare a raw material solution. Use a metering pump to draw the raw material liquid and the acylation liquid respectively, inject the two materials into a T-shaped mixer through a syringe for mixing, and then enter the microchannel reactor (inner diameter 0.5mm, length 6m) for reaction, the reaction time is 60min, where T The type mixer is placed in a constant temperature tank to control the temperature at 0°C, and the reaction tube is placed in the constant temperature tank to control the temperature at 35°C. The reacted product enters the T-type mixer again, and the other water is injected into the mixer through the meterin...
Embodiment 2
[0022] Put the stirred reactor into a low-temperature tank, add 15g of nitrobenzene, then add 4.5g of anhydrous aluminum trichloride, and finally add 2.5g of propionic anhydride, control the temperature at 0°C, and react for 10 minutes to prepare acylation liquid. Add 15g of nitrobenzene into a stirred reactor, and then add 1.5g of 2-methylnaphthalene to prepare a raw material solution. Use a metering pump to draw the raw material liquid and the acylation liquid respectively, inject the two materials into a T-shaped mixer through a syringe for mixing, and then enter the microchannel reactor (inner diameter 0.5mm, length 6m) for reaction, the reaction time is 60min, where T The type mixer is placed in a constant temperature tank to control the temperature at 0°C, and the reaction tube is placed in the constant temperature tank to control the temperature at 35°C. The reacted product enters the T-type mixer again, and the other water is injected into the mixer through the meteri...
Embodiment 3
[0024] Put the stirred reactor into a low-temperature tank, add 15g of nitrobenzene, then add 2.5g of anhydrous aluminum trichloride, and finally add 1.2g of acetyl chloride, control the temperature at 0°C, and react for 10 minutes to prepare acylation liquid. Add 15g of nitrobenzene into a stirred reactor, and then add 1.5g of 2-methylnaphthalene to prepare a raw material solution. Use a metering pump to draw the raw material liquid and the acylation liquid respectively, inject the two materials into a T-shaped mixer through a syringe for mixing, and then enter the microchannel reactor (inner diameter 0.5mm, length 6m) for reaction, the reaction time is 60min, where T The type mixer is placed in a constant temperature tank to control the temperature at 0°C, and the reaction tube is placed in the constant temperature tank to control the temperature at 35°C. The reacted product enters the T-type mixer again, another water is injected into the mixer through the metering pump, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com