Method for preparing 5-hydroxymethyl furfural from glucose
A technology of hydroxymethylfurfural and glucose, which is applied in the field of preparing 5-hydroxymethylfurfural from glucose, can solve the problems of unfriendly environment, high energy consumption for separation, high price, etc., and achieve low toxicity, environmental friendliness, simple preparation process, The effect of cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The preparation method of solid acid catalyst in following embodiment comprises the steps:
[0025] a. ZrOCl 2 ·8H 2 Dissolve O in deionized water, add HZSM-5 and stir to suspend, in which ZrOCl 2 ·8H 2 The ratio of O to deionized water is 1g: 100mL;
[0026] b. Add ammonia water dropwise to the material obtained in step a to stabilize the pH at 8 to 9, then age for 8 to 12 hours and filter to remove Cl - ;
[0027] c. Dry the material obtained in step b at 100-120° C. for 7-9 hours;
[0028] d, use the material obtained in step c with 0.5~1M H 2 SO 4 Vulcanize for 2 to 3 hours;
[0029] e. Calcining the material obtained in step d at 500-600°C for 2.5-3.5 hours to obtain the solid acid catalyst, expressed as S / Z x -HZSM, where S means SO 4 2- , Z means ZrO 2 , x is ZrO 2 Ratio to mass ratio of HZSM-5.
Embodiment 1
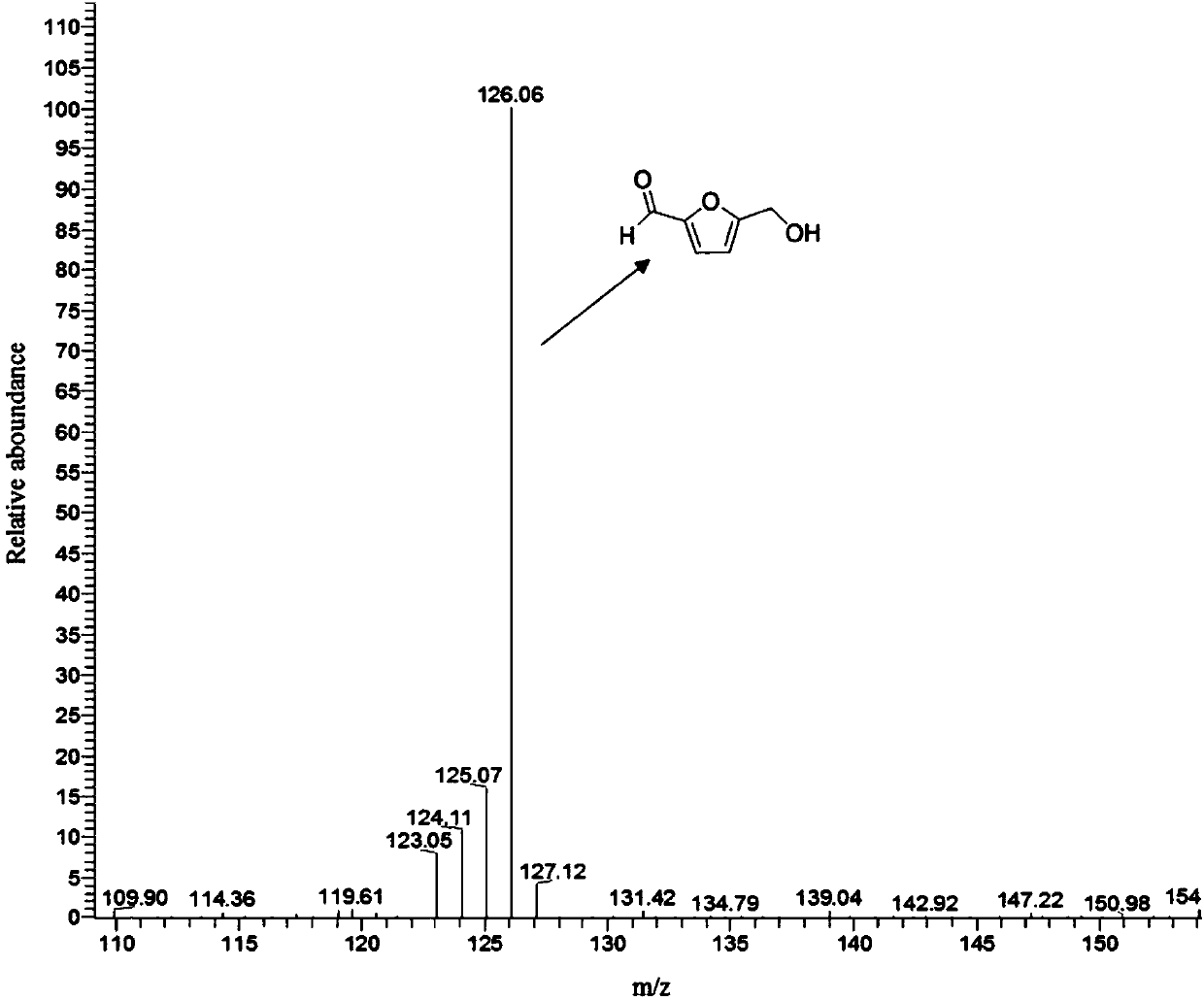
[0031] 0.15g glucose, 0.05gS / Z 0.05 -HZSM, 1.5mL 25%wt NaCl aqueous solution and 3.5mL ethyl acetate were added to the autoclave, heated to 195°C at a stirring speed of 450rpm and reacted for 2h to obtain a reaction phase and an extraction phase, and the extraction phase was taken for detection. According to gas chromatography analysis, the calculated yield of 5-hydroxymethylfurfural was 36.61%.
Embodiment 2
[0033] 0.3g glucose, 0.1gS / Z 0.05 -HZSM, 3mL 25%wt NaCl aqueous solution and 7mL ethyl acetate were added to the autoclave, heated to 195°C at a stirring speed of 450rpm and reacted for 2h to obtain the reaction phase and the extract phase. Analysis, the calculated yield of 5-hydroxymethylfurfural was 38.13%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com