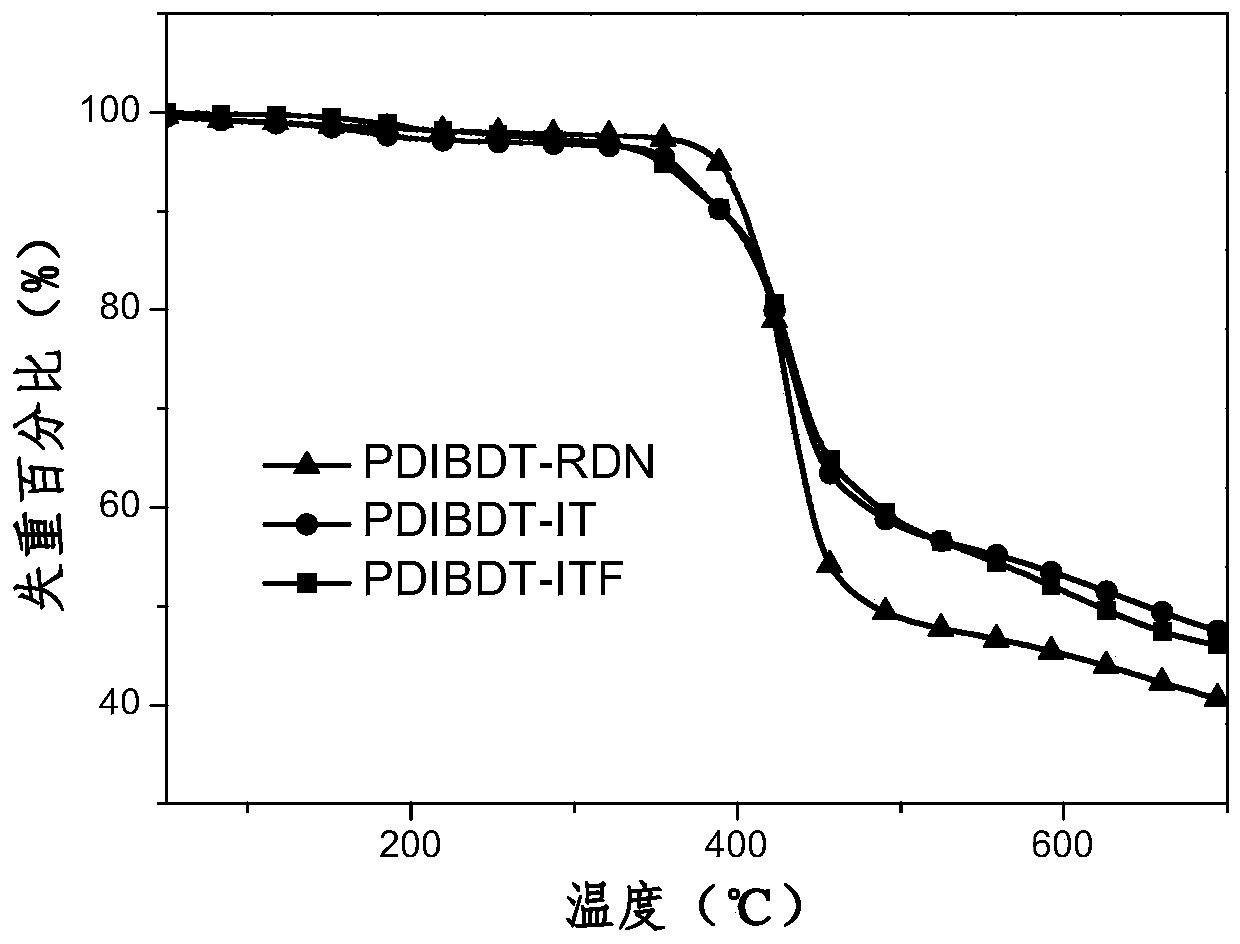
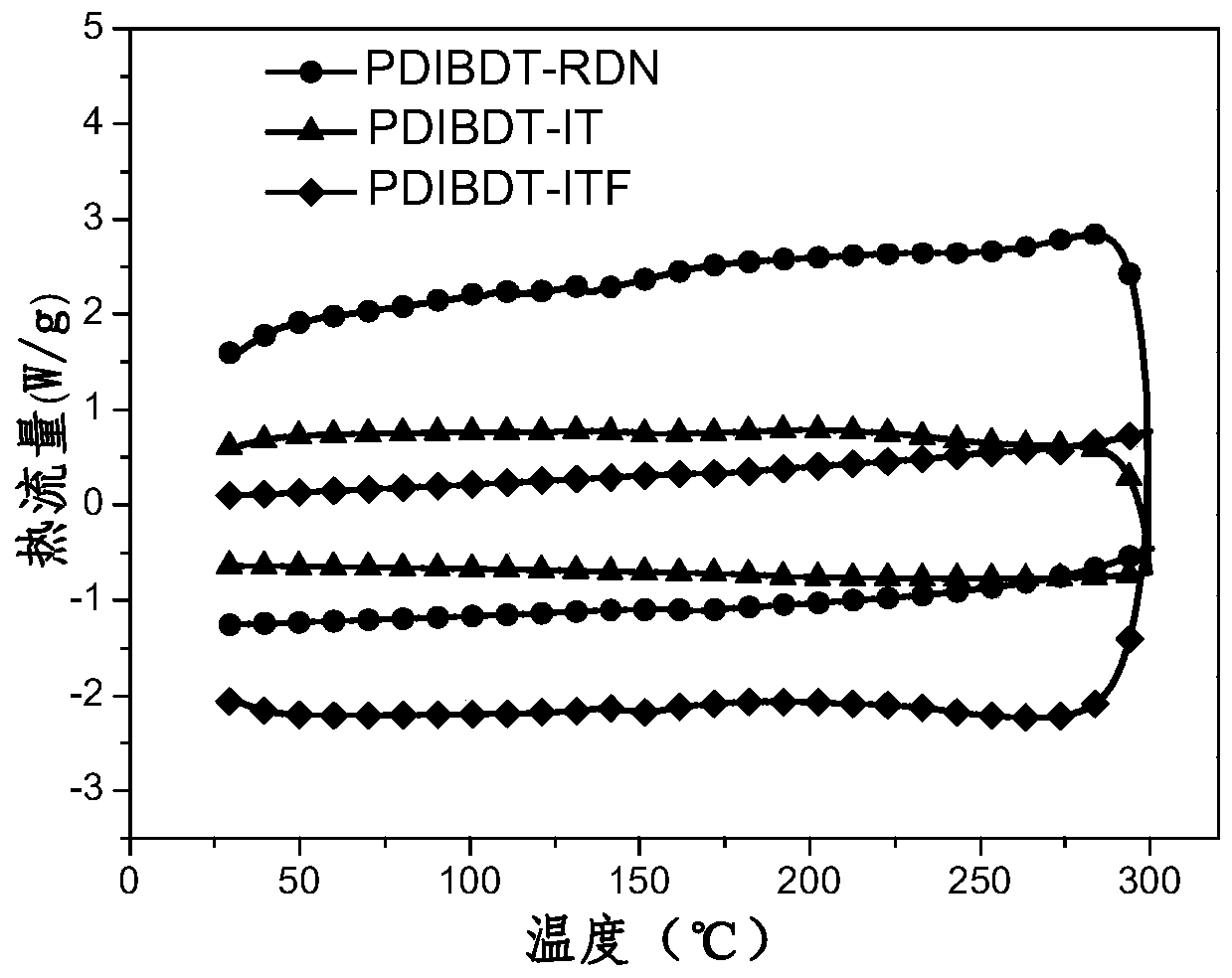
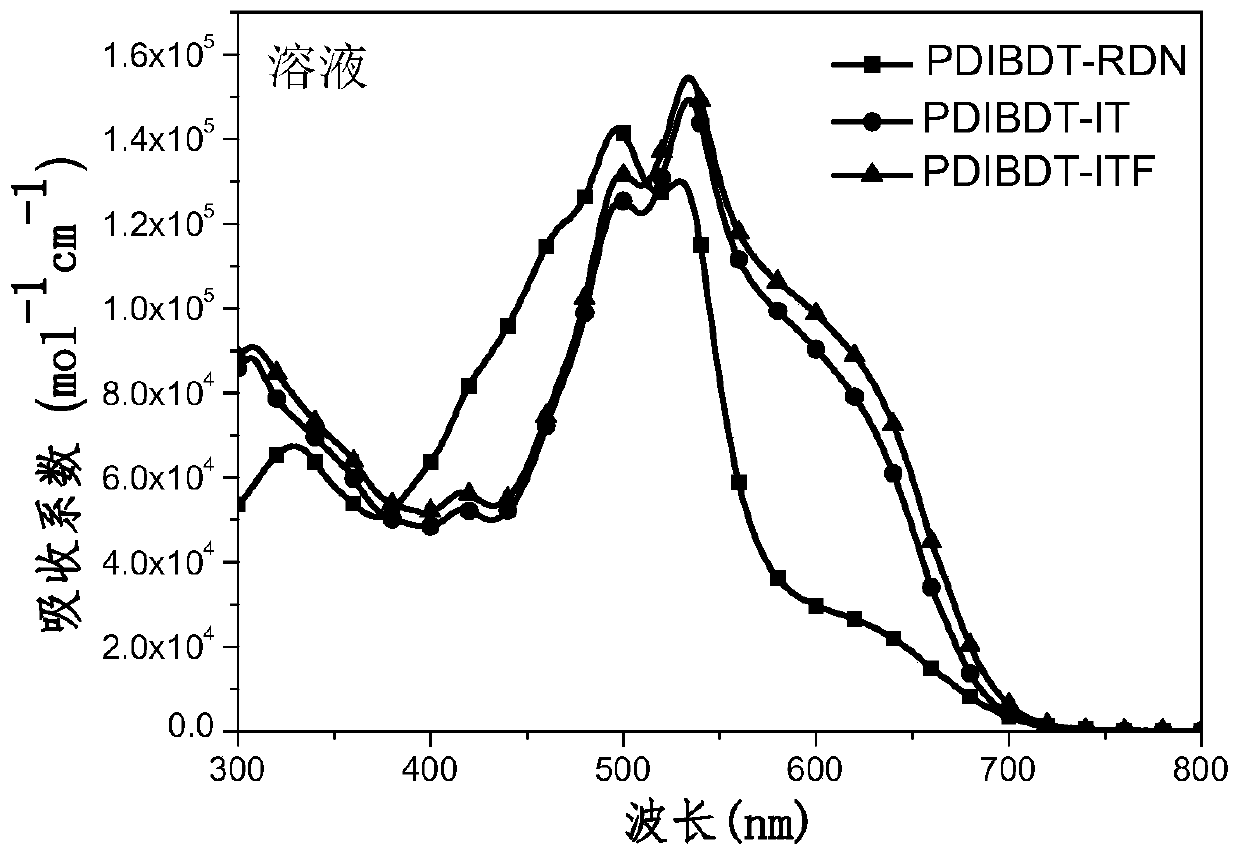
A kind of organic small molecule material containing benzodithiophene and its preparation method and application
A benzodithiophene and small molecule technology, applied in the field of organic solar cells, can solve the problems of weak absorption, affecting the improvement of organic solar cell devices, strong aggregation, etc., and achieve improved short-circuit current density, excellent device effect, and good thermal stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1, 4,8-bis((triisopropylsilyl)ethynyl)benzo[1,2-b:4,5-b']dithiophene (3).
[0041] A 250.0ml dry three-necked round-bottomed flask with a stirring bar was passed into argon, and under the protection of argon, compound 1 (ethynyltriisopropylsilane, 6.31g) and 75.0ml of dry THF were injected through a syringe. , the solution was clear and transparent, and then the reaction bottle was placed at -78°C for half an hour at a constant temperature, and n-BuLi (15.2ml, 2.5M) was added dropwise, and there was no obvious change during this process. After constant temperature reaction for 2.5hrs, add reactant 2 benzo[1,2-b:4,5-b']dithiophene-4,8-dione (grass green crystal, 2.54g), and slowly rise to room temperature React for 2.5hrs, add anhydrous SnCl 2 (17.50 g) was reacted overnight to finally obtain a light brown clear solution. During processing, the reaction liquid was directly transferred to a one-necked flask, and the solvent was spin-dried, and the column chromat...
Embodiment 2
[0042] Example 2, ((2,6-bis(trimethyltin)benzo[1,2-b:4,5-b']dithiophene-4,8-diyl)bis(alkyne-2,1 -diyl))bis(triisopropylsilyl)(4).
[0043] Weigh the reactant 3 (3.86g) in a 250.0ml dry two-necked round-bottomed flask with a stirrer, and pump it through a vacuum double-row tube three times each, and place it in a flask filled with argon protective gas. Add TMEDA (tetramethylethylenediamine, 3.25g) and dry THF (90.0ml), the solution is light yellow, stir at room temperature, after the solid reactants are completely dissolved, place the reaction solution at -78°C for half an hour , and then began to add n-BuLi (11.7ml, 2.5M) dropwise. When it was added dropwise to 8.0ml, the solution became turbid and a yellow solid was precipitated. After constant temperature reaction for 2.0hrs, inject Me with a syringe 3 SnCl (35.0ml, 1.0M), the solution became clear immediately, slowly rose to room temperature and reacted overnight. When processing, pour 100.0ml deionized water into the fla...
Embodiment 3
[0044] Example 3, 5,5'-(4,8-bis((triisopropylsilyl)ethynyl)benzo[1,2-b:4,5-b']dithiophene-2,6- Diyl)bis(4-(2-ethylhexyl)thiophene-2-carbaldehyde)(6).
[0045] Weigh compound 4 (2.0g, 2.28mmol) and 5-bromo-4-(2-ethylhexyl)thiophene-2-carbaldehyde (2.42g, 7.99mmol) in a 75.0ml dry pressure tube equipped with a stirring bar , add 60.0ml of dry toluene, and then bubble the long needle with argon for 15min to ensure that the pressure tube is exhausted and filled with argon inert gas. Then weigh the catalyst Pd (PPh 3 ) 4 (263.5mg, 10% eq) was quickly added to the pressure-resistant tube, and the long needle port stayed in the pressure-resistant tube for a period of time to ensure that the entire pressure-resistant tube was filled with argon gas, and the stopper was quickly covered and tightened, and then the reaction liquid It was heated to 110° C. under stirring and reacted overnight. After cooling to room temperature, transfer the reaction solution directly to a single-necked...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com